Abstract
Abstract
Introduction: We have undertaken this study to find the clinical, radiological, pathological and surgical outcome of patients with insular gliomas operated in our institute over an 11year period.
Material and Methods: Surgical outcome of the conservative safe resection is similar compared to the gross total resection is the hypothesis of the study. So, retrospective analysis of cases of insular glioma treated in a tertiary care institute from 2001 to 2011 was done.
Results: There were 65 male (67.7%) and 31 female patients (total 96 patients) with male: female ratio of 2.1: 1. The median age at diagnosis 36 years. The most common presentation was seizures seen in 70 patients (72.9%), followed by headache. Of the 96 cases 52(54.2%) were on the right and 44(45.8%) on left. Majority of our patients in this study underwent conservative resections with only 7 undergoing >80% resection and 89 underwent <80>80% resection was 18.8 months mean survival and for those < 80>
Conclusion: Insular gliomas are a unique group of gliomas in terms of its location and spread to surrounding lobes. In contrast to most studies, insular gliomas were of high grade in our study. Conservative resection to relieve mass effect, followed by adjuvant therapy is a safe and effective treatment for these critically located gliomas.
Keywords: Insula, Glioma, Seizures.
Introduction
The insular lobe is a functionally complex structure, harbouring peculiar anatomical and vascular features and specific neuronal connectivity with surrounding cerebral structure. It is thought to play roles in autonomic sensation, gustatory function, olfaction, memory, drive, auditory– vestibular function, and the motor integration and motor planning of speech in the dominant hemisphere.[1],[2],[3],[4],[5] The insula is also associated with cardio regulatory and vasomotor functions, pain perception, and bio-behavioural dysfunction characteristics of schizophrenia.[6]
Given the potential involvement (and impairment) of these essential neural networks, tumours affecting the insula may present with a variety of ill-defined symptoms[7] and also controversy persists as to which treatment strategy is appropriate for patients with insular gliomas and how intervention can affect patient outcome. Observation, stereotactic biopsy, radiosurgery, and microsurgical removal have all been proposed,[8],[9] In a recent epidemiological study by Duffau and Capelle,[10] insular glioma accounted for up to 25% of all low grade gliomas and 10% of all high grade gliomas.
Historically insular gliomas were considered inoperable and handled with stereotactic biopsy and radiotherapy or no further treatment.[11] Yasargil and colleagues[12] reported an extensive surgical series of limbic and par limbic intrinsic tumors (including insular gliomas), and they demonstrated for the first time that microsurgery is possible for tumors occupying that region without critical neurologic deterioration.[12]
We have undertaken this study to find the clinical, radiological, pathological and surgical outcome of patients with insular gliomas operated in our institute over an 11 year period.
Materials and Methods
Retrospective analysis of cases of insular glioma treated in our institute from 2001 to 2011 was done. Data of patients having undergone surgery for insular glioma was initially obtained from operation theatre records followed by detailed study of medical records of these patients from the medical records section of the institute. The data regarding the demographic profile, clinical presentation and operative notes were obtained from the files. The pre and post-operative radiology images and histopathology were reviewed. Follow up data was obtained from the files, outpatient examination and through telephonic interviews.
Statistical Analysis
Statistical analyses of the data were performed using the software IBM SPSS 19.0 version. Frequency distribution, Chi Square and Kaplan Meir chart were the statistical parameters used to analyse the data.
Results
Demographic data
There were 65 male (67.7%) and 31 female patients (total 96 patients) with male: female ratio of 2.1: 1. The median age at diagnosis 36 years. The most common presentation was seizures seen in 70 patients (72.9%), followed by headache. Of the 96 cases 52(54.2%) were on the right and 44(45.8%) on left. 81(84.3%) of the cases underwent transcortical and 15(15.7%) underwent transsylvian approach for resection of the insular glioma. 8 cases (8.3%) were graded as low grade and 88(81.7%) were high grade gliomas. Of the 96 cases 54.2% were on the right and 45.8% were on the left side.
Clinical Presentation
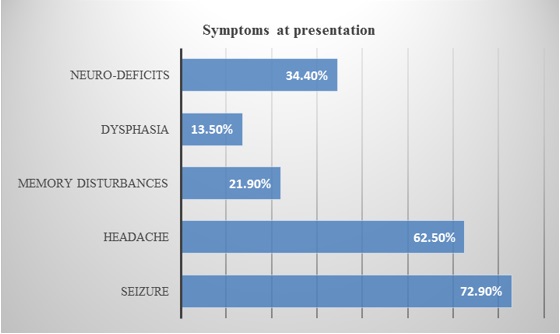
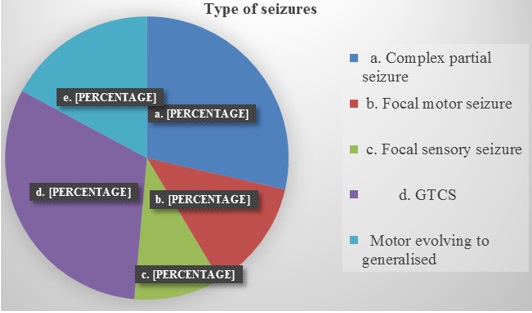
The most common presentation was seizures seen in 72.9% patients, followed by headache (Bar diagram 1). Pie diagram 1 is showing the type of seizure at the presentation. 62.5% of patients presented with headache of which 88% had raised intracranial pressure type of headache along with papilledema.
 |
Click here to view |
Bar diagram 1: Shows presenting symptoms of the patients. Seizure is the most common presentation among them (72.9%).
 |
Click here to view |
Pie diagram 1: Showing the type of seizure at the presentation. Generalized tonic clonic seizure is the most common type which accounts for 31% of seizures.
Radiology
Preoperatively all patients were evaluated with CT and/or MRI. Ninety two of the patients underwent CT. Seventy four were evaluated with MRI.
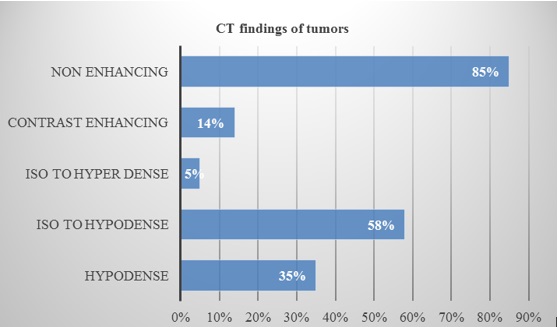
CT and MRI images were studied in detail regarding the heterogeneity of the tumour, extent of involvement, enhancement pattern, post-operative and follow up residue/recurrence. CT findings were given in the bar diagram 2.
 |
Click here to view |
Bar diagram 2: Showing CT scan features of insular gliomas.
MRI was used regularly for preoperative assessment except for initial part of this decade when MRI was used sparingly. A total of 74 cases of the 96 were evaluated with MRI.
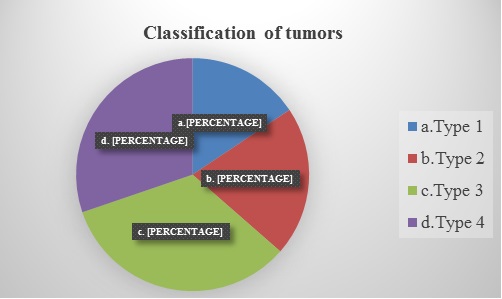
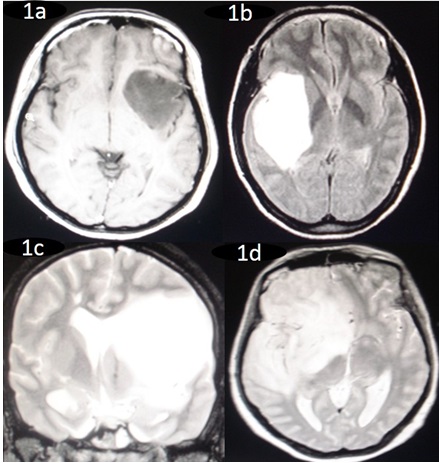
Tumours were classified radio logically depending on the anatomical locations (Oz yurt classification) into 4 types: Type1-Tumour restricted to insular region (Type 1);
Type 2-Tumour involving the insula and additionally operculae (frontal, temporal, parietal); Type 3- Tumor involving the insula, operculum and paralimbic structures (temporopolar, frontoorbital); Type 4-tumor Additional involvement of the limbic structures.(Pie diagram 2). Figure 1 shows the different anatomical locations of the tumors.
 |
Click here to view |
Pie diagram 2: Showing the percentage of tumours in the different anatomical locations.
 |
Click here to view |
Figure 1: Preoperative magnetic resonance imaging (MRI) showing 1a. Tumour restricted to insular region (Type 1), 1b.Tumour involving the insula and additionally temporal and parietal operculae (Type 2), 1c. Tumor involving the insula, operculum and para-limbic structures (Type 3) and 1d. Tumour Additional involvement of the limbic structures (Type 4). Figure 1 shows the different anatomical locations of the tumors.
Surgical approach
There were 2 approaches used for resection of insular glioma, transsylvian and transcortical. 81(84.3%) of the cases underwent transcortical and 15(15.7%) underwent transsylvian approach for resection of the insular glioma.
Extent of resection
The extents of resection were quantified as total, subtotal, partial and temporal decompression based on operative surgeons impression and post-operative imaging. A > 80% resection is considered as gross total decompression, 50-80% as subtotal and < 50>
Table 2: Showing the extent of tumour resection
|
Extent of resection Total decompression Subtotal decompression Partial decompression Anterior temporal lobectomy |
05(05.2%) 30(31.2%) 19(19.7%) 42(44.9%) |
Complications
Immediate Post-operative deficitsImmediate deficits seen as a result of surgery was seen in 21 patients.(Table 3)
Table 3: Showing Immediate Post-operative deficits
|
Post-operative deficits Facial Faciobrachial paresis Faciobrachial and speech disturbance Speech disturbance Hemiparesis Dense hemiplegia B/L 6th nerve |
1 5 1 4 6 3 1 |
However in 18 patients the deficits were transient and all of them had improved at discharge or 3 months follow up.
Histopathology
The operative specimens were processed and subjected to histopathological analysis by the neuropathology department in the institute and graded according to the WHO classification. WHO classification graded glioma into 4 grades. Grade 1 and 2 are classified as low and 3 and 4 were classified as high. In our study 8 cases (8.3%) were graded as low grade and 88(81.7%) were high grade gliomas
Adjuvant therapy
Patients with LGG were managed expectantly with serial neuroimaging except for 1 case of oligodendroglioma grade 2 who was advised adjuvant treatment in view of the residual lesion all the high grade gliomas were referred for adjuvant treatment.
Follow Up
Follow up was available for 70 patients. The mean follow up was 18.2 months, median follow up of 10.5 months with range of 1-76 months.
At follow up patients were evaluated with imaging which showed residual lesion in 20 patients and recurrence in 10 patients. Karnofsky performance score was done for patients at follow up. There were 46 patients with KPS of 100-90, 5 patients with score of 90-80, 10 with score of 80-70, 4 with 70-60 and 5 with poor score <50>
Recurrence
10 patients at follow up had recurrence of lesion of which 8 underwent reexploration.2 patient opted against second surgery and hence managed conservatively.
Survival analysis
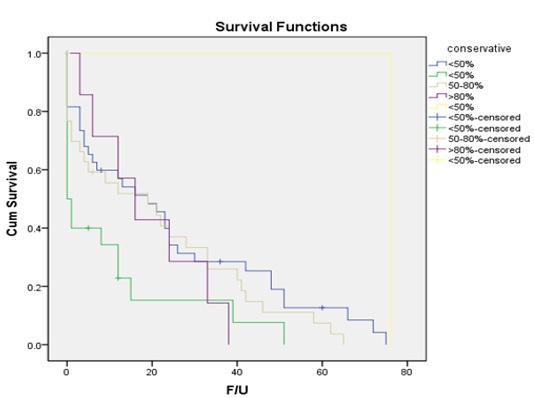
The overall mean survival was 20.3 months and median survival 12 months. Majority of our patients in this study underwent conservative resections with only 7 undergoing >80% resection and 89 underwent <80>80% resection was 18.8 months mean survival and for those < 80>
 |
Click here to view |
Fig. 2: Kaplan-Meier chart showing survival of patients depending on the resection of the tumor.
Discussion
There have been considerable controversies regarding the optimal treatment of insular glioma because of its complex neurovascular anatomy and physiology. Insular gliomas are the most frequent intrinsic tumour in the insular region, and they accounted for up to 25% of all low-grade gliomas (LGGs) and 10% of all high-grade gliomas (HGGs) in a recent epidemiologic study.[10]
The demographic profile of patients in our study was found to be similar to various other studies with median age of 36 years and male to female ratio of 2.1:1.[13],[14],[15]
Medically intractable epilepsy is the most common presenting symptom of patients with insular gliomas, especially those with low grade gliomas.[16] About 64% to 98% of patients with insular tumours suffer from intractable epilepsy, among whom simple and complex partial seizures accounted for 33 to 69% of cases and generalised seizures 23% to 29% of cases.[4],[12],[17],[18]Our study revealed that 72.9% of the patients presented with seizures and it was the most common presenting symptom with simple and complex partial seizures accounting for 51% and generalised 49% respectively. The semiology of seizures presenting in our study reiterates the fact that insular functional anatomy has diverse characters with manifestation as temporal lobe or frontal lobe epilepsy, simple partial seizures with respiratory, viscero-sensitive, or oroalimentary features.
Objective neuropsychometry done to evaluate cognitive disturbances in our patients with insular glioma revealed impairment of dorsolateral prefrontal cortex,, temporal, orbitofrontal and global areas. These tests revealed impairment in domains of memory especially verbal learning and memory, executive functions and mental processing speed. Also there was impairment of motor speed. Similar impairments for insular glioma patients were identified in learning and memory, executive functioning and processing speed in clinical article by Adam S Wu.[13]
We used both CT and MRI wherever available to define extension of the tumour, classify and decide surgical intervention required. We used the new classification of insular tumours by Emin Ozyurt.[18]Since in our study vast majority of the lesions were high grade a statistically significant correlation between the imaging features and grade of lesion could not be predicted.
The insula appears to be a preferential site for the growth of low-grade gliomas.[10] The representative surgical series reported that low grade gliomas accounted for 36 to 74% of insular tumours. Various other large series[4] ,[14]of insular glioma have shown low grade gliomas to be predominant histologic type. There was marked variation in the pathologic nature of lesion in our study as compared to various other studies. There were 88 cases of high grade and 8 cases of low grade glioma. There was statistically significant difference in the occurrence of high grade glioma in our study which casts doubt on the thinking that low grade histology is unique to insula.
Surgical Treatment and outcome
Surgery for intrinsic brain tumors will quickly relieve neurological signs and symptoms related to the lesion’s mass effect. The potential benefits of a tumor resection need to be balanced against the risk of incurring a new postoperative deficit. A significant new deficit will have an adverse impact on the patient’s quality of life, which may well outweigh any prolongation of survival. Iatrogenic worsening of the functional status may not only diminish the patient’s quality of life, but also his or her survival. This latter issue is of particular importance with respect to surgery for tumors located in or close to eloquent brain structures. Consequently, a conservative approach to the treatment of insular tumors, that is, obtaining a tissue diagnosis followed by radiation treatment or chemotherapy, as indicated by the tumor’s histological features, has been recommended by some authors.[8],[9]
Sanai and colleagues[4] have enrolled 104 patients, including 60% with LGGs and 40% with HGGs. In their work, patients with LGGs resected over 90% had a 5-year OS rate of 100%, whereas those with lesions resected less than 90% had a 5-year OS rate of 84%. In the same context, patients with HGGs resected over 90% had a 2-year OS rate of 91%, whereas those with lesions resected less than 90% had a 2-year OS rate of 75%. In our study the overall mean survival was 20.3 months and median survival 12 months. The short survival values seen in our study may be due to the fact that vast majority of our cases were high grade lesions.
Our data confirm that surgical treatment for insular gliomas carries a substantial neurological morbidity.[11] ,[14],[15]There were 21/96 patients with neurologic deficit (21.9%) in the immediate post-operative period which improved to normal in all but 3. Only 3/96 had permanent neurologic deficit (3%). In the study by Duffau[18] constituting 51 cases of insular glioma immediate neurologic deficit was seen in 59% of the patients, so also vanaclocha[15],[19] in his study of 23 cases of insular glioma showed immediate deficit of 26% and Sanai[4] reported deficit of 14% in his study of 104 cases of Insular glioma. Our study thus had good outcome in terms of postoperative deficits.
Conclusion
Insular gliomas are a unique group of gliomas in terms of its location and spread to surrounding lobes. Seizures and raised intracranial pressure are the most common presenting features. In contrast to most studies, insular gliomas were of high grade in our study.
Conservative resection to relieve mass effect, followed by adjuvant therapy is a safe and effective treatment for these critically located gliomas.
Conflict of Interest: None.
References
- ^ Duffau H. Surgery of Insular Gliomas. InIntracranial Gliomas Part I-Surgery 2018;30:173-185. Karger Publishers.
- ^ Michaud K, Duffau H. Surgery of insular and paralimbic diffuse low-grade gliomas: technical considerations. J neuro oncol 2016;1;130(2):289-298.
- ^ Wang Y, Wang Y, Fan X, Li S, Liu X, Wang J, Jiang T. Putamen involvement and survival outcomes in patients with insular low-grade gliomas. J neurosurg 2016;126(6):1788-1794.
- a, b, c, d, e Sanai, N., M.Y. Polley, and M.S. Berger, Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg 2010;112(1):1-9.
- ^ Ture, U., et al., Topographic anatomy of the insular region. J Neurosurg 1999. 90(4):720-733.
- ^ Makris, N, Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res, 2006;83(2-3):155-171.
- ^ Isnard, J, Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia, 2004;45(9):1079-1090.
- a, b Mehrkens, J.H, Long term course of WHO grade II astrocytomas of the Insula of Reil after I-125 interstitial irradiation. J Neurol 2004. 251(12): p. 1455-64.
- a, b Shankar, A. and V. Rajshekhar, Radiological and clinical outcome following stereotactic biopsy and radiotherapy for low-grade insular astrocytomas. Neurol India 2003;51(4):503-506.
- a, b, c Duffau, H. and L. Capelle, Preferential brain locations of low-grade gliomas. Cancer, 2004;100(12): 2622-2626.
- a, b Ebeling, U. and K. Kothbauer, Circumscribed low grade astrocytomas in the dominant opercular and insular region: a pilot study. Acta Neurochir (Wien), 1995;132(1-3):66-74.
- a, b, c Yasargil, M.G., Tumours of the limbic and paralimbic systems. Acta Neurochir (Wien), 1992;118(1-2):40-52.
- a, b Özyurt, E, New Classification for Insular Tumors and Surgical Results of 40 Patients. Neurosurg Quarterly 2003;13(2):138-148.
- a, b, c Vanaclocha, V., N. Saiz-Sapena, and C. Garcia-Casasola, Surgical treatment of insular gliomas. Acta Neurochir (Wien), 1997;139(12):1126-34; discussion 1134-1135
- a, b, c Duffau, H, The insular lobe: physiopathological and surgical considerations. Neurosurg 2000. 47(4): p. 801-10.
- ^ Kim, Y.H. and C.Y. Kim, Current surgical management of insular gliomas. Neurosurg Clin N Am 2012. 23(2):199-206.
- ^ Moshel, Y.A, Resection of insular gliomas: the importance of lenticulostriate artery position. J Neurosurg 2008;109(5):825-834
- a, b, c Duffau, H., A personal consecutive series of surgically treated 51 cases of insular WHO Grade II glioma: advances and limitations. J Neurosurg 2009;110(4):696-708.
- ^ Hentschel, S.J. and F.F. Lang, Surgical resection of intrinsic insular tumors. Neurosurg 2005. 57(1):176-183.





