Abstract
History of laboratory accreditation in the world including India is very poor. The concept of accreditation started at the period of world war and thereafter, to main quality of supplies provided to shoulders. In India, first institute created for this purpose was Indian Standards Institution. At present, after WTO and GATT, the quality of laboratory are maintained to ease the export and import facilities using quality test results under the concept "One world, one health". In India, the laboratory accreditation and instrument calibration are guided by NABL, the nodal authority, under Govt. of India. An attempt has been taken to give precise and accurate informations without altering so much terms and explanation of ISO/IEC/NABL. Even somewhere words and meanings are kept as it is not to alter the inner meaning of cited authorities.
Keywords: Laboratory accreditation, ISO/IEC 17025:2005, ISO/IEC 17025:2017, NABL.
Introduction
In British dictionary, a laboratory is a facility that provides controlled conditions in which scientific or technological research, experiments and measurement may be performed. In 2017 as per ISO 17025, the "laboratory is body that performs one or more of the following activities: testing, calibration and sampling, associated with subsequent testing or calibration". In early, well known labs include Pythagoras lab, Louis Pasteur lab etc with their paintings where, presently specialized labs are created in mostly all subjects including language laboratory. At present, a test result of a sample done in an accreditated laboratory is well accepted in another country of the world. Like a test result done in NABL lab in Kolkata will be gladly accepted in America.
Accreditation literally means a credit, given by third party or a recognized body, attests technical competence within a lab, specified in scope of accreditation, adhered and operated under a documented quality system. In India, the only accreditation body is �National Accreditation Board for Testing and Calibration Laboratories (NABL)�. International Laboratory Accreditation Cooperation (ILAC) is the single global system consists of region-wise accreditation like India, Australia, New Zealand etc are under Asia Pacific Laboratory Accreditation Cooperation (APLAC). The NABL is under the APLAC requirements.
ISO (International Organization for Standardization) is an international standard forming body composed of representatives from different national organizations. Till January, 2018, total members are 162 from all over the world. Both the name and logo of ISO are registered trademarks and used in restricted manner. ISO along with International Electro-technical Commission (IEC) developed standards and terminology in electrical and electronic matters. India is member body/full member where Bureau of Indian Standards (BIS) is the standard body, having voting rights. Committee on Conformity Assessment (CASCO) is the ISO committee works on conformity assessment issues and develops policy and publishes standards thereof.
Different steps for a new standardization like ISO 17025:2017 starts Preliminary Work Item (PWI) by Preliminary Stage, New Proposal /New Work Item Proposal (NP/NWIP) by Proposal stage, Approved new Work Item (AWI), Working Draft (WD) by Preparatory Stage, Committee Draft (CD) by Committee Stage, Final Committee Draft (FCD) by Approval Stage, Draft International Standard (DIS), Final Draft International Standard (FDIS), Proof of a new International Standard (PRF) and finally International Standard (IS) by Publication Stage. There are also Enquiry, Review and Withdrawal Stages in the entire process.
Bureau of Indian Standards (BIS) was formerly known as Indian Standards Institution (ISI) is under Ministry of Consumer Affair, Food and Public Distribution, Govt. of India. It acts as pivotal role in World Trade Organization � Technical Barriers on Trade (WTO-TBT) to prevent unnecessary obstacles on trade. Whereas Quality Council of India (QCI) is an autonomous body along with constituent Boards are National Accreditation Board for Testing and Calibration Laboratories (NABL), National Accreditation Board for Certification Bodies s (NABCB), National Accreditation Board for Education and Training (NABET), National Board for Quality Promotion (NBQP) and National Accreditation Board for Hospitals and Healthcare Providers (NABH).
National Accreditation Board for Testing and Calibration Laboratories (NABL) is an autonomous body under the aegis of Department of Science and Technology, Govt. of India. It aims to provide third party assessment on quality and technical competence of testing and calibration laboratories of Government, Industry Association and Industry. NABL provides laboratory accreditation services in performing tests and calibration in accordance with ISO/IEC 17025. When a product tested once that becomes accepted everywhere is the ultimate goal. A chain continues on quality and competence from ILAC to APLAC, NABL to all NABL accredited labs. So a lab recognized by NABL is at par with world quality and is accepted through ILAC implemented countries.[1][2][3][4]
Scope of NABL Accreditation lies as below:
Testing Laboratories
Biological-
Chemical-
Electrical-Electronics-
Fluid flow-
Mechanical-
Non-Destructive-
Photometry-
Medical Laboratories
Clinical Biochemistry-
Clinical Pathology-
Haematology and Immunohaematology-
Microbiology and Serology-
Histopathology-
Cytopathology-
Genetics-
Nuclear Medicine (In vitro test only)
Calibration Laboratories
Electro �Technical-
Mechanical-
Fluid Flow-
Thermal-
Optical-
Radiological-
Proficiency Testing Provider (PTP)
Testing-
Calibration-
Medical-
Inspection-
Reference Material Producers (RMP)
Chemical Composition-
Biological and Clinical Properties-
Physical Properties-
Engineering Properties-
Miscellaneous Properties-
Classes of tests in biological testing discipline:
- Food and Agricultural products-
- Drugs and Pharmaceuticals-
- Water-
- Environment and Pollution-
- Biocides-
- Cosmetics and Essential Oils-
- Industrial Cultures-
- Seed Testing-
- Plants and Plant Materials-
- Molecular Analysis-
- Cell Culture-
- Resistance to Microbial Attack-
- Biological Tests on other Misc. test items-
- Biopesticides and Biofertilizers-
- Toxicology-
- Identification/Enumeration of Microbial Pathogens-
- Residue analysis-
- Veterinary Testing-
- Nutraceuticals and Functional Foods-
- Nutritional Supplements-
- Animal Food and Feed-
- Antimicrobial Activity Products-
- AYUSH Products-
- Biological Monitoring-
- Biologicals Derived Pharmaceuticals-
- Cosmetics and Essential Oil-
- GM Products-
- Marine/Aquaculture Food Products-
- Medical Accessories and Surgical Products-
- Molecular Analysis-
- Wild Life Forensic-
XVIII: Veterinary Testing
It includes specified tests in biochemistry, haematology, cytopathology, histopathology, serology, parasitology, virology, immunology etc. As per different authors book as mentioned, the following tests may come under the category Veterinary testing.
A) Veterinary clinical biochemistry: (Serum/Plasma):
- Total protein, albumin, globulin, bilirubin, A:G ratio, Fibrinogen, Bilirubin.
- Immunological like C-reactive protein, Total Ig, IgG, IgM, IgA, IgA.
- SGOT, SGPT, AP, arginase, lipase, creatine phosphokinase, lactic dehydrogenase (LDH). d) Lipid Profile: Lipase, Cholesterol, Triglycerides, HDL-Direct, LDL-Direct, VLDL-Calculated.
- Electrolytes like, K, Na, Ca.
- Haemostatic disorders like Bleeding, Coagulation, Thrombin and Prothrombin time.
B) Veterinary clinical haematology:
- Blood smear staining with Leishman, Giemsa, Methylene blue, Wright.
- Special stain: Gram stain, Z-N stain for TB, polychrome Methylene blue for anthrax,
- Blood parameters: Hb, TEC, TLC, DLC, PCV, ESR, Platelets count, MCV, MCHC, MCH etc.
C) Veterinary cytopathology: FNAC, Flow cytometry, Karyotyping, flow cytometry
D) Histopathology:[5]
-
- Biopsy
- Histochemistry for collagen, reticular, elastic fungus, amyloid. AFB, spirochaete, goblet cell, mast cells, iron etc.
- Immunohistochemistry and cytochemistry
- In situ hybridization
�
E) Serology and Immunology: Antigen and antibody tests including immunopathology
F) Clinical parasitology: Coproscopical, ecto parasite, endoparasite, haemoprotozoa
G) Virology: CPE
H) Clinical pathology:
Kidney function test: Total protein, Creatine, Creatinine, Uric acid, Urea, Electrolytes, Calcium and Phosphorous.
Urine Analysis
-
-
- Physical: Specific gravity,
- Chemical: pH, protein (Roberts test), Acetone (Ross test), Blood (Occultest), Bilirubin (Gmelin test), Urobilinogen (W.D. test).
- Microscopical:
-
Cast: Epithelial, waxy, pus cell, hyaline, granular, fat cast.
Crystal: Uric acid, calcium oxalate, hippuric acid, calcium carbonate, triple phosphate, ammonium urate, bilirubin, tyrosine, leucine, cystine.
Thyroid function test: T3, T4, TSH.
Hormone: FSH, LH, Prolactin
Pancreatic function test: Serum amylase and Glucose (Random and fasting).
Liver function test: Birubin from serum (van den Bergh test), SGOT, SGPT, AP, Sorbitol dehydrogenase (SDH), Arginase (horse). BSP excretion test, Serum protein, Urobilinogen.
Cardiovascular disease tests: CPK, SGOT, LDH.
CSF fluid examination: colour, specific gravity, coagulation, protein, enzymes, cell count, sugar, culture, Leishman stain.
Semen analysis: Physical, Microscopical.
Mastitis test:
-
-
- Bromothymol Blue (BTB) test,
- Modified California Mastitis Test (MCMT) by RFCL
- Somatic Cell Count (SCC) of milk etc
-
�
Pregnancy test: Per rectal and urine tests
I. Microbiology: Culture and Antibiotic Sensitivity test:
-
-
- Anaerobic culture of blood
- Eye: conjunctiva, vitreous humor fluid
- Vaginal/Rectal sample
- Synovial fluid (CSF)
- Seminal fluid
- Catheter culture
- Milk culture
-
J. Stress detection:
-
-
- Total Antioxidant Status (TAS)
- Superoxide Dismutase (SOD)
- Glutathione Peroxidase (GSH-Px)
-
k. Post-mortem of animals:[6]�[7]Large, small, avian, lab and wild animals may be used for PM.
m. Molecular pathology: PCR, RT-PCR
n. Forensic pathology: Finger printing of primates, wildlife
o. Imaging[11]
- X-ray
- USG
- EEG
- Phonandoscopes
- Pleximeters and percussion-hummers
- Electronic stethoscope
- Ophthalmoscopes
- Blood pressure monitors
- Otoscopes
- Laryngoscopes
- Oesophagoscopes
- Tracheo-scopes.
ISO Structure
�
�
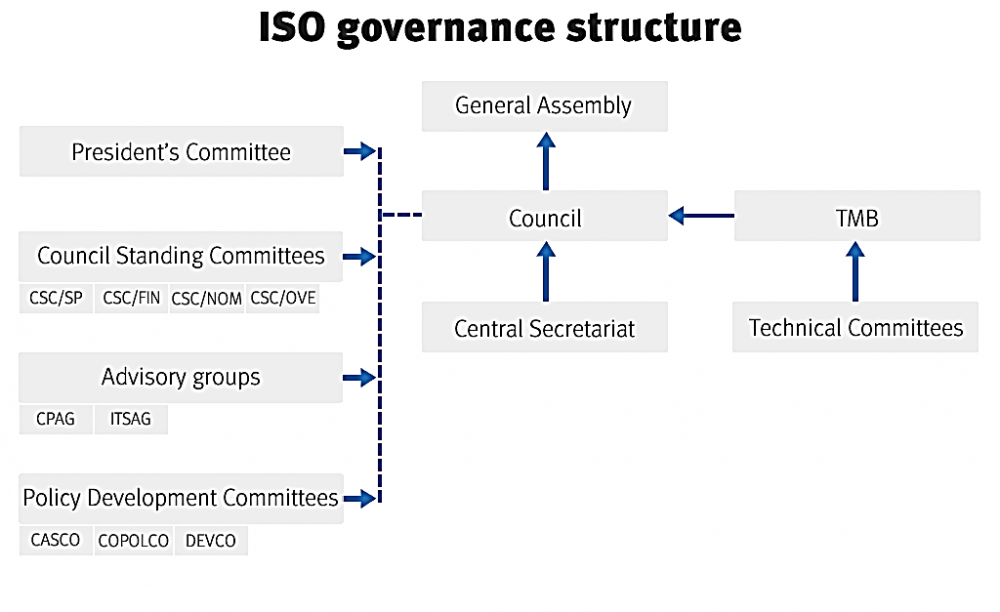 |
Click hear to view |
Internationally Recognized Accreditation Bodies:
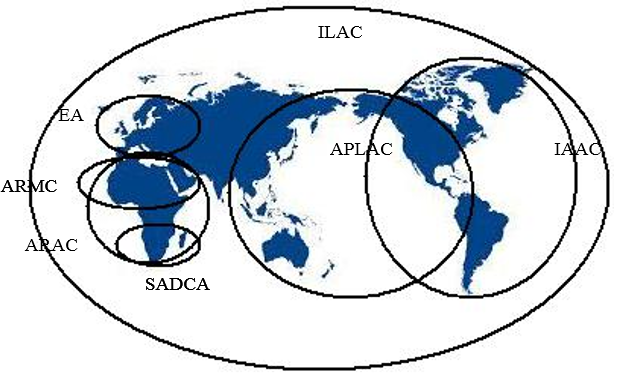 |
Click hear to view |
�
ARAC � African Regional Accreditation Cooperation
IAAC - Inter-America Accreditation Cooperation
APLAC - Asian Pacific Laboratory Accreditation Cooperation
EA � European Cooperation for Accreditation
ILAC � International laboratory Accreditation Cooperation
SADCA � South African Development Community Accreditation ARMC-Arabic Accreditation Cooperation
Common ISO Standards Related to Laboratory Accreditation
ISO: 9001 Quality Management
ISO: 13485 Medical devices
ISO: 14001 Environmental Management
ISO: 14064 Green House Gases
ISO: 15189 Medical Laboratories Quality Systems
ISO: 15700 Service Quality Management System
ISO: 16001 Social Accountability
ISO: 17021 Conformity Assessment
ISO: 17025 Testing and Calibration Laboratories
ISO: 18001 Occupational Health and Safety Management Series
ISO: 22000 Food Safety Management
ISO: 26000 Social Responsibility
ISO: 27001 Information Security Management
ISO: 31000 Risk Management
ISO: 37001 Anti-bribery Management System
ISO: 45001 Occupational health and safety
ISO: 50001 Energy Management
|
Clause. No. |
ISO/IEC 17025: 1999 |
ISO/IEC 17025: 2005 |
ISO/IEC 17025: 2017 |
|
1 |
Scope |
Scope |
Scope |
|
2 |
Normative references |
Normative references |
Normative references |
|
3 |
Terms and Definitions |
Terms and Definitions |
Terms and Definitions |
|
4 |
Management requirement |
Management requirement |
General requirement |
|
5 |
Technical requirement |
Technical requirement |
Structural requirement |
|
6 |
- |
- |
Resource requirement |
|
7 |
- |
- |
Process requirement |
|
8 |
- |
- |
Management requirement |
�
The new standard (ISO 17025:2017) is structured as below:
Scope: The document specifies the general requirements for the competence, impartiality and consistent operation of laboratories and is applicable to all organizations performing laboratories activities irrespective of number of personnel.
Normative References: It includes the latest reference like ISO 17025:2017 with all other relevant references as per ISO.
Terms and Definitions: The ISO and IEC maintain terminological databases available www.iso.org/obp and www.electropedia.org
General Requirements
Impartiality: It includes independence, freedom from conflict of interests, freedom from biasness, lack of prejudice, neutrality, fairness, open mindedness, even handedness, detachment and balance. The laboratory shall not allow commercial, financial or other pressures to compromise impartiality.
Confidentiality: The laboratory shall treat all information confidential unless asked by law.
Structural Requirements: The laboratory shall be a legal entity and have management for overall responsibility. It shall define and document the range of laboratory activities conforms to its document. Externally provided laboratory activities are not encouraged on an ongoing basis. The laboratory may be permanent, temporary or mobile facilities. A laboratory shall have an integrated and effective organization and management, competent and responsible personnel.
Resource Requirements
General: A laboratory shall have available personnel, facilities, equipment, systems and support services to manage and perform laboratory activities.
Personnel: Laboratory personnel both internal and external shall have competence to perform laboratory activities and evaluate the significance of deviation. The laboratory shall communicate the duties, responsibilities and authorities to their laboratory personnel including development, modification, verification and validation of methods, analysis of results, report, review and authorization of results. However, there should have specific procedures and records in relation to determining the competence requirements, selection, training, supervision, authorization and monitoring of competence of personnel.
Facilities and Environment Conditions: The facilities and environmental conditions including contamination, interference or adverse influences towards laboratory activities as per specification for laboratory testing will be implemented, monitored, periodically reviewed and documented. If the laboratory activities are outside of the permanent control, the facilities will be at the main lab.
Equipment: The laboratory shall have a procedure for handling, transport, storage, use and planned maintenance of equipment for proper functioning and to prevent contamination and deterioration. Related items like measuring instruments, softwares, measurement standards with measurement accuracy, reference materials, reference data, reagents, consumables or auxiliary apparatus etc responsible for correct performance of equipment will be ensured. Out of service equipments will be separated, labelled and coded and the reference value and correction values needs to be updated after calibration.
Records shall be retained for equipment which can influence the laboratories include identity of equipments including software and firmware versions, manufacturers name, serial number, evidence of verification, current location, calibration related informations, documentation of reference materials, results, acceptance criteria, relevant dates, period of validity, maintenance plan with details of any damage, malfunction, modification, repair etc.
Metrological Traceability: It is �property of a measurement result whereby the result can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty�. The laboratory shall ensure that measurements results are traceable to the International System of Units (SI) through calibration provided by a competent laboratory or by certified values of certified reference material or direct realization of ensured SI units.
Externally Provided Products and Services: Before recommendation, the lab should ensure suitability of the products and services for their lab. The laboratory shall have procedure and retain record for defining, reviewing and approving laboratories requirement for such products and services, evaluation, selection, monitoring of performance and re-evaluation of external providers, conformity criteria with lab requirement.
Process Requirements
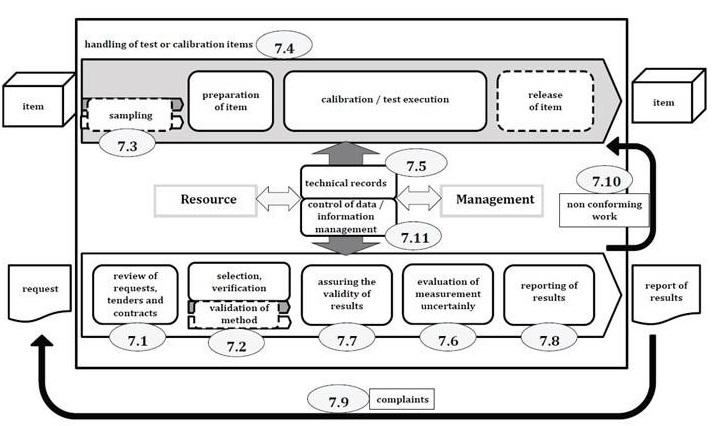 |
Click hear to view |
A possible schematic representation of the operational processes of a laboratory
Review of Requests, Tenders and Contracts: The laboratory should have a procedure of defined requirement by customer, well documented and understood. Also laboratory should have capability and adequate resources to meet requirement. Customers approval needed f external service is used. Out of date or inappropriate test requested by customer will be informed the up to date test methods. Any deviation should be amended accordingly with customer and lab.
Selection, Verification and Validation of Methods: The appropriate methods, procedures, evaluation of uncertainty measurement, statistical techniques for data analysis, supporting documents like instructions, standards, manuals, reference data etc shall be readily available to personnel. In absence of customers desired test method, an appropriate method will be chosen and informed. The methods will be used either published in international, regional or national standards, or by reputable scientific texts or journals, or as specified by the manufacturer of equipment or laboratory developed or modified validated methods in labs. The laboratory developed, non-standard or other used methods should be validated by one or combination of using reference standards or reference material evaluation, comparison of results achieved by other validated methods, inter laboratory comparison along with consideration of influencing factors, controlled parameters, measurement of uncertainty etc.
Sampling: Sampling is the selection of suitable sample for study where samples represent the representative part of a whole. There should be selection of samples or site, sampling plan, preparation and treatment of sample during sampling method. But records of data should include reference of sampling method, date and time of sampling, number, amount, number of samples, identification personnel conducting sampling, equipment, environment or transport conditions, diagram for sampling location, deviation, addition or exclusion for sampling method and plan etc.
Handling of Test or Calibration Items: The laboratory shall have a procedure for transportation, receipt, handling, protection, storage, retention, disposal or return of test or calibration items, protection of test or calibration test integrity and interest of lab and customer. Precaution shall be taken to avoid deterioration, contamination, loss or damage of item during handling, transporting, storing or waiting and preparation of testing or calibration etc. Deviation of testing from customer of lab ends or doubt about suitability for testing or calibration along with disclaimer in the test report should be recorded accordingly. If some samples have to be stored or conditioned under specified environmental conditions, all such shall be maintained, monitored and recorded.
Technical Records: The laboratory shall keep all relevant technical records, previous amendments with date of alteration, name of person amendment done etc. This shall include date and identity of person responsible for each laboratory activity and for checking data, results along with original observation, data and calculations.
Evaluation of Measurement Uncertainty: Understanding of theoretical principles and practical experience of the testing along with sampling will be considered. For testing laboratory for a particular test method whose results are established and verified, there is no need to evaluate measurement uncertainty for each result if done in controlled manner. But for calibration labs, measurement uncertainty should be done considering all possible contribution and sampling.
Ensuring the Validity of Results: The laboratory shall have a procedure for monitoring the validity of results includes: use of reference or quality control materials, functional checks of testing equipment, use of working standard with control charts, intermediate checking, replicate tests, retesting of retained items, correlation of results, review of reported results, inter laboratory comparison, testing of blind samples etc.
Reporting of Results: Each results shall be reviewed and authorized prior to release where each report shall have a title, name and address of lab, location including permanent, associated or mobile facility, unique identification, customer name and contact, name of used method, condition of item, date of receipt, sampling, performance, issue of report, units of results, deviation if any, identity of person authorizing report, clear identification when results obtained from external providers. The report also state that the results apply to the samples as received. Specific environmental conditions, opinion, interpretations and additional information may be required by authorities or group of customer may be provided.
In calibration certificates, environmental conditions, measurement uncertainty, metrological traceability, results before and after adjustment or repair, statement of conformity with requirement or specification, recommendation of calibration interval as per customer asked for will be considered. However, reporting sampling, reporting statement of conformity, reporting opinion and interpretation will bear the needful informations.
In amendment to reports, any change information shall be clearly identified and reason may be incorporated. After reissue, a statement �Amendment to Report, serial number.� may be made. When new report issued, a uniquely identified and previous reference will be inserted.
Complaints: The process of handling complaints will include description of receiving process, validation, investigation and action decision, tracking and recording, acknowledging, progress report, final report of investigation, action taken with preventive measures and official closure notice of complain will be done. Actual person involved in testing shall not be included in the complain execution.
Nonconforming Work: The laboratory shall retain records of nonconforming work and actions including responsibilities and authorities for management of nonconforming work, actions including work halting, withhold of report, evaluation, decision on acceptability, recalling and notification to customer and resumption of work and corrective action for nonconforming work.
Control of Data and Information Management: This system will be useful for collection, processing, recording, reporting, storage or retrieval of data.
8 Management System Requirements
Options (Option A and Option B)
Option A: As a minimum, the management system of a laboratory should have management system documentation, control of the same, control of records, actions to address risk and opportunity, improvement, corrective actions, internal audits and management reviews.
Option B: Applicable to labs who has a management system in accordance of ISO 9001.
Management System Documentation (Option A): Laboratory shall establish, document and maintain policies and objectives, commitment and access to management system documentation.
Control of Management System Documents (Option A): All document of a laboratory will be approved, periodically reviewed, amended, identified but unintended use of obsolete documents will be prevented.
Control of Records (Option A): All the records will be responsible for control of identification, storage, protection, back-up, archive, retrieval, retention time and disposal of records.
Actions to Address Risks and Opportunities (Option A): More opportunities will be provided to lab to extend the purpose and objectives of the laboratory.
Improvement (Option A): The laboratory shall identify and select opportunities for improvement and implement any necessary action in a continual way.
Corrective Actions (Option A): Nonconformity will be addressed properly and will be documented.
Internal Audits (Option A): The laboratory shall plan, establish, implement and maintain audit programme with its own audit criteria, report of audit result, implementation of correction and corrective actions, records etc for internal audit.
Management Reviews (Option A): Different inputs like change in internal and external issues, objective fulfilment, suitability of policies and procedures, status of action taken, outcome of recent internal audit, corrective actions, assessment by external body, change in type and range of lab activities, customer and personnel feedback, complaints, effectiveness of implemented improvements, adequacy of resources, results of risk identification, outcome of assurance of validity results as well as outputs like effectiveness of management system and its processes, improvement of laboratory activities, provision of required resources and any need for change will be discussed in management review.
Transition: After the end of 2020, all the NABL laboratories will be under new standard that is ISO/IEC 17025:2017. So the labs whose renewal is before end of 2020, may apply for new standard implementation to avoid complication in future. The below mentioned tables will be helpful for adaptation of new standard.
For ISO 17025: 2017 the below mentioned four items should be taken care of.[3]�[4]
Table 1: Documents kept for accreditation
|
5.3 |
Range of laboratory activities |
|
5.5C |
Procedure for lab activities and validity of results |
|
6.2.2 |
Competence requirements including training etc |
|
6.3.2 |
Facilities and environment conditions |
|
7.8.6.1 |
Decision rule |
|
7.8.7.1 |
Basis of opinion, interpretation by authorized person |
|
7.9.1 |
Process of complaints |
|
8.1.1 |
Management system |
|
8.2.2 |
Policy and objectives of lab |
�
Table 2: Procedures kept for accreditation
|
6.2.2 |
Competence requirements including training etc |
|
6.2.5 |
Procedures for selection, training etc of personnel |
|
6.4.3 |
Outside equipment use |
|
6.6.2 |
External provided products and services details |
|
7.1.1 |
Review of request, tenders and contracts |
|
7.4.1 |
Disposal or return of test or calibration items |
|
7.7.1 |
Monitoring the validity of results |
|
7.10.1 |
Non conformity of results etc |
�
Table 3: Retain records for accreditation
|
6.2.5 |
Procedures for selection, training etc of personnel |
|
6.3.3 |
Monitor control & record of environmental conditions |
|
6.4.13 |
Equipment details |
|
6.6.2 |
External provided products and services details |
|
7.1.8 |
Records of review including significant change |
|
7.2.1.5 |
Method verification and revision |
|
7.2.2.4 |
Validation procedure, result, validity, fitness etc |
|
7.3.3 |
Sampling data |
|
7.8.7.3 |
Dialogue with customer for opinion and interpretation |
|
7.10.2 |
Non conformity work and actions |
|
8.7.3 |
Nature of NC, causes, actions, corrected results |
|
8.8.2 |
Audit programme |
|
8.9.2 |
Inputs to management review |
|
8.9.3 |
Outputs from management review |
�
Table 4: Records for accreditation
|
7.4.3 |
Deviation, suitability of item with disclaimer |
|
7.4.4 |
Item storage or conditioned under specifications |
|
7.5 |
Technical records like result, report, amendments etc |
|
7.7.1 |
Monitoring the validity of results |
|
7.9.3 |
Complain handling |
�
Conclusion
It may be concluded that after the new version, ISO/IEC 17025:2017, all the laboratories have to implement the new version within 2020. The following main steps are needed to follow as below:
- Do not wait till the last day of 2020.
- Training to the personnel who will be responsible for transition and implementation
- Analyse the gap between new and old versions of ISO/IEC 17025.
- Implement the new management system.
- Meet the general, structural, resource and process requirements as per new version.
Acknowledgements
The authors are thankful to NABL to keep up to date with the ISO/IEC 17025: 2005 and ISO/IEC 17025: 2017 and West Bengal University of animal and Fishery Sciences, Kolkata-700037, India for necessary permission.
Conflict of Interest: None.
References
- a, b ISO 17025 General requirements for the competence of testing and calibration laboratories 1999.
- a, b ISO 17025 General requirements for the competence of testing and calibration laboratories 2005.
- a, b, c, d ISO 17025 General requirements for the competence of testing and calibration laboratories 2017, 1-30.
- a, b, c, d NABL training manuals for ISO/IEC 17025: 2005 and ISO/IEC 17025: 2017 conducted by NITS, Noida and QCI respectively.
- ^ Luna LG. Manual of histologic staining methods of the Armed Forces Institute of Pathology 3rd ed. Mc. Graw Hill Book Co, London, 1968: p124.
- ^ Khatry SD. Postmortem appearances in animals. 1st ed. SDK, Bhilwara, 1999: P1.
- ^ Chauhan HVS, Roy S. Poultry diseases diagnosis and treatment. 3rd ed. New Age International (P) Limited, New Delhi, 2007: p729.
- ^ Benjamin MM. Outline of Veterinary Clinical Pathology. 3rd ed. The Iowa State University Press, Ames, Iowa, USA, 1961: p 3.
- ^ Kumar M, Sarin SK. Biomarkers of diseases in medicine. Current trends in Science.2009: 416.
- ^ Roy BK. Veterinary pharmacology and Toxicology. 3rd ed. Kalyani Publishers, Ludhiana. 2008. P 507.
- ^ Minimum standards of Veterinary Education (MSVE). Published by Veterinary Council of India (VCI), under the Department of Animal Husbandry, Dairy and Fishery, Govt. of India. 2017. p12.





