Clinicopathological, morphological and ki-67 proliferative index in oral squamous cell carcinoma in a tertiary care teaching hospital
Article Type : Null
Author Details:
Volume : 4
Issue : 1
Online ISSN : 2581-3706
Print ISSN : 2581-3714
Article First Page : 1
Article End Page : 8
Abstract
Introduction: Oral cancers are neoplasms which are malignant and affect the structures or tissues of the mouth. These malignant neoplasms can begin as lesion which is primary or can be from a distant site which can be metastatic. It can also be an extension of some adjacent tumoural area.[1]In developing countries, oral cancer is one of the most important cause of morbidity and mortality. Oral cancer is sixth most common cancer worldwide, ranking eighth in developed countries and third in the developing world.[2] A higher incidence is reported in Southeast Asia. Of all the cancers in the Indian subcontinent, this carcinoma accounts for almost 40%.[3]
Aims: To study clinicopathological, morphological and ki-67 proliferative index in oral squamous cell carcinoma.
Materials and Methods: This study is carried out in Department of Pathology, Mahatma Gandhi Medical college and Hospital, Sitapura, Jaipur, Rajasthan from 1 June 2015 to 31st May 2017. Institutional ethical committee approval was obtained prior to study.Informed consent was obtained from the patients before enrolment into the study.
Statistical methods: sensitivity, positive predictive value, negative predictive value, and specificity was calculated. The study was a Hospital based observational study.
Results: A total of 50 histologically diagnosed cases of “oscc”, with clinicopathological parameters and Immunohistochemical marker comprising of Ki-67 was studied. Out of non-invading 12 cases, 9(18%) had “Ki-67 Labeling Index” as high and 3 cases (6%) showed low labeling index.Out of 34(68%) high grade cases, in 29 cases (58%), Ki-67 expression was high and 5 cases (10%) had low expression. Also, out of remaining 16(32%) low grade cases, 7 cases (14%) showed high expression of Ki-67 and 9 cases (18%) showed low expression and show statistically significant p value.
Conclusion: Oral squamous cell carcinoma is significantly associated with elderly age group and with male sex.Ki-67 expression was statistically significant when compared with histological differentiation. This indicates that Ki-67 staining may be of use in individual tumor diagnosis and prognosis. In the histopathological assessment of the severity of oral squamous cell carcinoma, Ki-67 marker proves to be a clinically useful supplement.
Hence we propose that Ki-67 is a useful marker of proliferation. It can be used for the diagnosis of oral epithelial dyspalasias which have tendency to undergo malignant transformation
Keywords: Ki-67, Oral squamous cell carcinoma, Histology.
Oral cancers are neoplasms which are malignant and affect the structures or tissues of the mouth. These malignant neoplasms can begin as lesion which is primary or can be from a distant site which can be metastatic. It can also be an extension of some adjacent tumoural area. Oral cancer is sixth most common cancer worldwide, ranking eighth in developed countries and third in the developing world.[2] A higher incidence is reported in Southeast Asia. Of all the cancers in the Indian subcontinent, this carcinoma accounts for almost 40%.[3] Oral cavity cancers are the most common cancers in males in India. Also, in females, it is the third most common cancer.[4]
Oral cavity includes lips, oral tongue (anterior 2/3rd), floor of mouth, buccal mucosa, gingiva, hard palate, retromolar trigone, base of the tongue, tonsillar area & soft palate. The most common affected sites in the oral cavity are buccal mucosa followed by the floor of mouth.[5]
In oral cavity, the most common malignant tumours is squamous cell carcinoma. It comprises 90% to 95% of the oral malignancies.[6] Most cases are seen in males in the age of 50 years. Also there is an increasing incidence of oral malignancies among women and younger patients. Some cases have also been documented in children, particularly in the tongue.[7][8]
The etiological factors can be multiple factors like smoked tobacco and smokeless tobacco, diet and nutrition, viruses, alcohol, immunosuppression and chronic infection.[6]Some independent risk factors includes incomplete dental status and poor hygiene.[9]
In the development of malignancy, tobacco and alcohol has been reported as an important cofactor. In India, the principal cause of oral carcinoma is habit of chewing pan (this mixture contains betel nut leaves rolled with lime, catechu and tobacco) which results when buccal mucosa is in prolonged contact of the carcinogen.[5]It commonly affects men who had a previous history of high tobacco consumption, poor orodental hygiene, alcohol and diet which is not rich in vegetables and fruits. Also, secondary infection caused by the human papilloma virus (HPV) and Herpes Simplex Virus (HSV-1) adds to the high risk for the development of cancer.[10]
The premalignant lesions like “leukoplakia”, “erythroplakia” and “submucous fibrosis” may leads to the development of OSCC. They are recognized as oral precancerous lesions, and has histopathological features of epithelial dysplasia which ranges from mild to severe.[11] The most common oral precancerous lesion is
“leukoplakia” that has a high rate of malignant transformation i.e. from 0. 6% to 18%. The transformation rate from leukoplakia to oral cancer is based on the microscopic examination of dysplasia and degree of dysplasia.[12]
Squamous Cell Carcinoma can be:-[13]
Grade I: “Well differentiated”
Grade II: “Moderately differentiated”
Grade III: “Poorly differentiated”
Grade IV: “Anaplastic”
The prognosis of OSCC patients who are treated early is better with as high as 80% 5 years survival rates.[14]
The TNM system of the International Union Against Cancer is used for staging of neoplasm that can assess tumor size and lymph node involvement. The Imaging techniques adds to the accuracy of primary tumor (T), and nodal (N) staging.[15]
Molecular markers which investigates the heterogeneity of tumor can be useful in explaining the relapse as well as the mechanism of occurrence of OSCC.[16]
Molecular based assays has increased in the recent years but histopathology will always remain the gold standard for therapeutic and diagnostic use. Now days, the globally available tool that complements the analysis of histopathology by detecting the gene expression at the protein level is immunohistochemistry.[17]
The main characteristics of cancer cells are potential to replicate which is limitless and self renewal. If proliferation is uncontrolled, it indicates that the cells can acquire more alterations at cellular level which contribute to a full malignant phenotype. Ki-67 protein expression is related strictly with cell proliferation. It is present in all active phases of the cell cycle (i.e. G1, S, G2, and mitosis), but it is absent in resting cells (G0 phase). Hence, by representing growth fraction in tumor it acts as a promising nuclear marker for the proliferation of cells in malignancies.[18]It is observed that high proliferative activity is always associated with a poor prognosis and the proliferation of cells always serves as a guide for the prognosis of malignancies.[19]
Carcinogenesis can occur in any area of mucous membrane exposed to carcinogens with the risk of developing a second or multiple primary carcinomas.[20] ,[21]Ki-67 antigen is the commonest immunohistochemical marker used to study the proliferative index of the cell.[22]
Ki-67 has emerged as a great marker and is used as the standard to assess cell proliferation as internal as well as external factors doesn’t influence it much. The nuclear expression of Ki-67 during a fixed period of the cycle shows that as a marker it has an advantage to examine mitotic activity.[23] After cells have crossed proliferative stage, the residual staining is less produced because Ki-67 has shorter half life.[24] Therefore it explains that the cell which has passed through that stage is in proliferative stage than being residual evidence of that cell.[25] The clinical course of the disease is correlated with positive Ki-67 cells.[26]
The widely used marker of cell proliferation is Ki-67 LI, i.e., the percentage of the cells in a tissue staining for this marker. Premalignant or malignant condition of oral cavity can be predicted by this marker.[27] Genes encode the key proteins which are associated with growth and proliferation.[14]
Thereby in this study, the morphological features, histological grading and staging of tumour helps in evaluating the prognosis of this neoplasia. Ki-67 immunohistochemical expression is a good marker of cellular proliferation.[14][28].
To study the incidence of oral squamous cell carcinoma encountered in the Department of Pathology in MGH, Jaipur. To study the correlation of grading and expression of Ki-67 in oral squamous cell carcinoma. To study dysplasia and malignant squamous cell carcinoma and its relation with proliferative activity and distribution of Ki-67.To study the prognostic and predictive value of Ki-67 in oral squamous cell carcinoma.
The study was a conducted from 1 June 2015 to 31st May 2017 at Department of Pathology, Mahatma Gandhi Medical college and Hospital, Sitapura, Jaipur, Rajasthan. Institutional ethical committee approval was obtained prior to study. Informed consent was taken from patients before enrolment into the study. A total of minimum 50 cases of patients presenting with lesion of oral cavity had been studied.
Eligibility Criteria
Inclusion Criteria: Premalignant and malignant cancers of oral cavity are included in the study.
Exclusion Criteria: Improperly fixed specimen, Autolysed tissues,Patients who refuse to give consent, Inadequate biopsy tissue, Recurrent tumors.
Study Procedure
Biopsy tissue was fixed in buffered 4% formaldehyde, embedded in paraffin, cut at 4 micron, and stained with hematoxylin and eosin (H&E).
Immunostaining for Ki-67 will be carried out. Following endogenous peroxide and protein blocking step, the slides will be incubated with primary antibodies. After brief washes, incubation in a cocktail of biotinylated rabbit anti mouse IgG/IgM for 30 minutes will be performed.
The present study was undertaken during a period of from 2015 to 2017. Formalin fixed specimens of oral biopsies were received in the pathology department of Mahatma Gandhi Medical College and Hospital.
Detailed microscopic examination of the tissue was done by the experienced pathologist to arrive at the Histopathological diagnosis.
Immunohistochemical stains performed by using paraffin sections of the (50 cases) original diagnostic samples with primary antibody MIB-1 against Ki-67 were received and evaluated as follows:
The results were compared with the previous histological diagnosis done with the routine H&E staining only.
The most common age of presentation for malignancy was between 40-69 years (66%). The most common age group for SCC was 60-69 years (30%) followed by 40-49 years (22%). Mean age was found to be 51±15. 5 years in cases of SCC.
The study comprises maximum number of cases were found to be in the age group of 40-69 years (66%), mostly being male (80%). Female comprised of only 20% of the patient. Thus, the male to female ratio in our study is 4:1.
Maximum number of the patients chewed tobacco and smoked(48%), followed by combined habit of smoking and alcohol (14%). The remaining (12%) each indulged in only smoking, tobacco chewing and all three habits together. Very few (2%) did not have any social habit.
In the present study, commonest site of Squamous Cell Carcinoma was tongue (52%) followed by buccal mucosa (30%).
Out of 50 cases of squamous cell carcinoma, 16 cases (32%) were well differentiated, 31 cases (62%) were moderately differentiated and only 3 cases (6%) were poorly differentiated.
Out of 50 cases, In 8 cases (16%) lymph node show reactive hyperplasia and 4 cases (8%) showed metastatic squamous cell carcinoma. Lymph node status could not be assessed in the remaining 38 cases (76%).
All the 50 cases were subjected to immunohistochemistry for Ki-67. Ki-67 positive expression was detected in all the cases. Out of total 50 cases 36 cases (72%) were found to have high Ki-67 expression and only 14 cases (28%) were found to have low Ki-67 expression.
Out of 50 cases the most common cancer was moderately differentiated carcinoma 31 cases (62%) followed by well differentiated carcinoma 16 cases (32%) and 3 cases of poorly differentiated carcinoma 3 cases (6%), out of these the maximum number of cases were seen in males 40 cases(66%) and only 10 cases (34%) cases in females.
Out of total 50 cases, Ki 67 was expressed highly in people between age group 45-84 with ta total of 36 cases of which 25 (50%) had high expression and 11 (22%) had low expression.while for age group less than 45 years only 11 cases (22%) had high expression and 3 cases has low [removed]6%). It was statistically significant with p value being 0.5187
Relation between KI-67 Expression and gender
Table 1: Relation Between KI-67 Expression & Gender
|
Gender |
KI-67 Expression |
P-Value |
|
|
High |
Low |
||
|
Female |
6 (12%) |
4 (8%) |
0. 3447 |
|
Male |
30 (60%) |
10 (20%) |
|
Relation between KI-67 Expression and TNM staging
Table 2: Relation between KI-67 Expression & TNM Staging
|
TNM Staging |
KI-67 Expression |
P-Value |
|
|
High |
Low |
||
|
pT1 pT2 pT3 |
9 (18%) |
3 (6%) |
0. 5193 |
|
N/A |
27 (54%) |
11 (22%) |
|
As to pathological staging, out of 50 cases, 38 cases (76%) had no information of the tumour invasion into adjacent structures because their small biopsy were received. Remaining 12 cases (24%) had tumors which did not invade adjacent structures (pT1, 2, 3). Out of non-invading 12 cases, 9(18%) show high
Ki-67 proliferative index and 3(6%) show low Ki-67 proliferative index. While in remaining 38 cases whose pathological tumour status is not known, 27 cases (54%) had high Ki-67 proliferative index and remaining 11 cases (22%) had low index. Pathological staging did not show any statistically significant p value.
Relation between KI-67 expression and histological grading
Table 3: Relation Between KI-67 Expression & Histological Grading
|
Histological Grading |
KI-67 Expression |
P-Value |
|
|
High |
Low |
||
|
MDSCC |
26 (52%) |
5 (10%) |
0. 0121 |
|
WDSCC |
7 (14%) |
9 (18%) |
|
|
PDSCC |
3 (6%) |
0 |
|
|
Two cells have value <5> |
|
|
|
In histological grading, 16 cases (32%) with well differentiated squamous cell carcinoma, 7(14%) had high Ki-67 proliferative index and 9 cases (18%) had low Ki-67 proliferative index. Out of 31 cases (62%) of moderately differentiated squamous cell carcinoma, 26 cases (52%) show high Ki-67 and only 5 cases (10%) had low Ki-67 LI.
Only 3 cases (6%) were poorly differentiated and had high
Ki-67 LI. The relationship of Ki-67 expression was statistically significant when compared with histological grading. (p value=0. 0121).
In the present study, the most common site of Squamous Cell Carcinoma was tongue (52%) in which maximum number of cases were of Moderately differentiated squamous cell carcinoma (36%) followed by well differentiated squamous cell carcinoma (14%). The second most common site in the present study was Buccal mucosa which also included maximum number of cases of moderately differentiated squamous cell carcinoma(14%) followed by well differentiated squamous cell carcinoma (12%).
Table 4: Relation Between Ki-67 Expression and TNM Staging
|
KI-67 Expression |
TNM Staging |
||||
|
N/A |
Stage I |
Stage II |
Stage III |
Stage IV A |
|
|
High |
27 (22%) |
3 (6%) |
3 (6%) |
0 |
3 (6%) |
|
Low |
11 (22%) |
1 (2%) |
1 (2%) |
1 (2%) |
0 |
Relation between gross morphology and Histological grading
Table 5: Relation Between Gross Morphology and Histological Grading
|
Gross Morphology |
Histological Grading |
|||
|
MDSCC |
PDSCC |
WDSCC |
Grand Total |
|
|
Nodular |
3 |
|
1 |
4 |
|
Ulcerative |
28 |
3 |
15 |
46 |
|
Grand Total |
31 |
3 |
16 |
50 |
Out of 50 cases of oral squamous cell carcinoma, maximum cases presented with ulcerative growth (46 cases)
in which maximum cases were diagnosed as moderately differentiated squamous cell carcinoma.
Relation between clinical features and Histological grading
Table 6: Relation between clinical features and histological grading
|
Clinical features |
Histological grading |
|||
|
MDSCC |
PDSCC |
WDSCC |
Grand Total |
|
|
D |
3 |
|
1 |
4 |
|
H |
7 |
|
4 |
11 |
|
D, H |
21 |
3 |
11 |
35 |
|
Grand Total |
31 |
3 |
16 |
50 |
On distributing the patients on the basis of clinical features, mostly patients presented with a complaint of both
dysphagia and hoarseness who were diagnosed as moderately differentiated squamous cell carcinoma
 |
Click here to view |
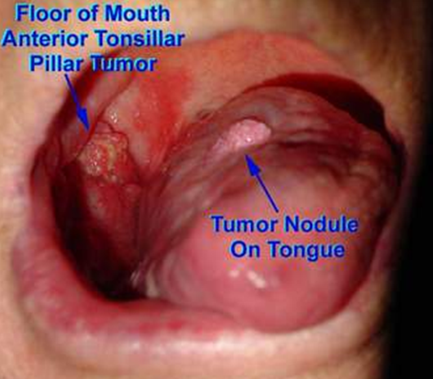
Fig. 1: A nodular and ulcerated lesion of OSCC on Floor of Mouth and Tongue
 |
Click here to view |
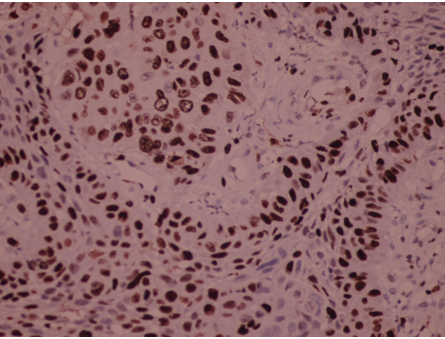
Fig. 2: Ki-67 immunopositivity in MDSCC (moderately differentiated squamous cell carcinoma) of Tongue (H & E,400x)
 |
Click here to view |
Fig. 3: Ki-67 poistive PDSCC(poorly differentiated squamous cell carcinoma) of Buccal Mucosa (H & E,400x)
 |
Click here to view |
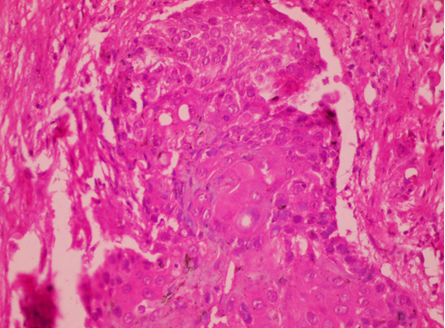
Fig. 4: Poorly differentiated squamous cell carcinoma of Buccal Mucosa (H&E 100x)
 |
Click here to view |
Fig. 5: Poorly differentiated squamous cell carcinoma of Tongue (H&E 100x)
 |
Click here to view |
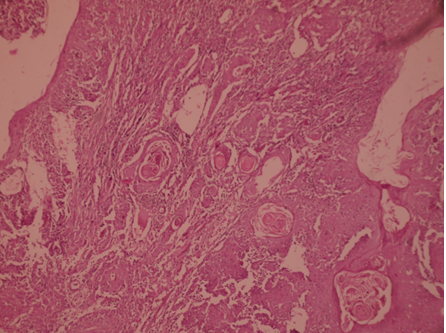
Fig. 6: Poorly differentiated squamous cell carcinoma of Buccal Mucosa (H&E 100x)
This study entitled “Clinicopathological, morphological and Ki-67 proliferative index in oral squamous cell carcinoma in a tertiary care teaching hospital” was done in the Department of Pathology, Mahatma Gandhi Medical College, Jaipur during the year 2015-2017 on the 50 cases. Although the lesions are easily accessible for clinical examination, their prognosis can be difficult to assess in the context to location variability, risk factors, histopathological and molecular aspects involved in their appearance and progression. Present study comprises of 50 cases of oral squamous cell carcinoma diagnosed on histopathology.
The aims of this study is assessing the Ki-67 marker and to correlate other prognostic factors such as age, sex, social habit, histological staging and histological grading with expression of these markers.
Distribution of Age Group
In the present study, the mean age of the patients of oral squamous cell carcinoma was 51±15. 5 years and range from 20 to 79 years. The patients was maximally distributed in the 40-69 years (66%) age group.
Sharmistha M Patel et al (2014)[9] observed the mean age of oral squamous cell carcinoma cases was 49±12. This was in accordance to our study.
Abhay R. Chandak et al (2011)[29] observed the mean age of oral squamous cell carcinoma cases was 53. 20 years.
According to a study conducted by Sieczka E et al (2011),[30] the mean age of presentation of oral cancer is 75 years ranging in age from 34 to 94 years in Asian population.
Distribution According to Gender
Our study showed maximum number of cases in male (80%). Female comprised of only 20% of the patient. Thus, the male to female ratio in our study is 4:1.
Males are affected more often than females because of heavier indulgence in both tobacco and alcohol habits in most countries. In India the highest rates of intraoral cancer may be found in women who chew tobacco heavily.[31]
In a study by Sharma P et al (2010)[32] on 80 patients of oral squamous cell carcinoma, men were more (68. 7%) than women (31. 2%).
A study by Doshi Neena P et al (2011)[33]on 80 cases of oral cancer, showed a prevalence of 61. 25% in males. Males were found to be in higher proportion due to high use of tobacco both in the form of chewing and smoking compared to females as the consumption of tobacco and tobacco products was less in the females.
A study carried out by L. P. Dragomir et al (2012)[34]on 34 oral squamous carcinomas, mostly age of patients was from 20-40 years (61. 8%), males being 85. 3% and smokers and/or alcohol consumers being 85. 3%.
In 80 cases of oral cancer, Khandelkar et al (2006)[35] studied that prevalence of cancer in males was 61. 25%. Males were found to be in higher proportion due to high use of tobacco both in the form of chewing and smoking compared to females as the consumption of tobacco and tobacco products was less in the females.
Distribution of Patients According to Social Habit and Anatomical Site
In our study group, maximum number (%) of patients chewed tobacco and smoked (48%), followed by combined habit of smoking and alcohol (14%). Tongue was the most commonest of cancer (52%) followed by buccal mucosa (30%).
This is in concordance with the study done by Sharmistha M Patel et al (2014)[9] on 39 cases who found that tongue was the commonest site of oral cancer followed by buccal mucosa.
In the study on 57 cases of oral cancer, Marcelo Gadelha Vasconcelos et al (2014)[36]found that the most common site of squamous cell carcinoma was tongue. The most frequent habit was combination of smoking and alcohol (42.1%), smoking alone was followed by it. (31.6%).
Muhammad Kashif et al (2015)[1] observed that in oral cavity, the most commonly involved site was tongue (mostly lateral border of tongue) (46%) followed by patients present with tumour on Buccal mucosa (6%). Also high proportion of patients with OSCC were habitual smokers and had a habit of pan chewing.
Halboub et al (2012)[37]also identified floor of mouth, tongue as the most common sites of oral cancer. Smoking (96.3%) and smokeless tobacco (91.8%) were identified as the major risk factors among various risk factors for causing oral cancer.
Whereas the study conducted by Tahir et al (2013)[38]is in contrast with the present study which reported that Buccal mucosa was the most common site of involvement which was seen predominantly in (32.4%) cases.
Also, the study conducted by Nemes et al (2008)[39]in Hungary reported that the floor of mouth was the most common site of involvement in OSCC patients.
Distribution According to Histological Grading
In the present study, Out of 50 cases of squamous cell carcinoma, maximum number of cases (62%) were moderately differentiated followed by well differentiated (32%). Only few cases (6%) were of poorly differentiated OSCC.
As considering the histological grading of OSCC, the study conducted Jerjes et al (2010)[40]stated that half of the patients were presented with MDSCC which is favour of with the present study.
“Well and moderately differentiated SCC” were the most common histological grade of oral SCC in young patient reported by Iype et al (2000)[41]and Iamaroon et al (2004).[42]On the other hand, in studies carried out by Effiom (2008),[43] poorly differentiated SCC was the most common grade of oral SCC.
Comparison between Gender and Histological Grade of OSCC
Chi-square test was applied to study the relation between gender and histological grade of OSCC and it was found statistically insignificant.
This is in accordance with study done by Muhammad Kashif et al (2015)[1]who observed, out of 127 patients mostly well differentiated and moderately differentiated tumours were seen (44.9%) whereas only few (10.2%) were poorly differentiated. Relation of gender and histological grade of OSCC was statistically insignificant.
Correlation of Ki-67 Expression and Histological Grade
Ki-67 marker is tested in all 50 cases. Out of total 50 cases, 36 cases (72%) were found to have high proliferation for Ki-67 and only 14 cases (28%) were found to have low proliferation.
In histological grading, the low grade (well differentiated) were 16 cases (32%), and high grade were 34 cases (68%) (moderately and poorly differentiated). Out of 34 (68%) high grade cases, 29 cases (58%) had high Ki-67 expression and 5 cases (10%) had low levels. Also out of remaining 16 (32%) low grade cases, 7 cases (14%) had high expression of Ki-67 and 9 cases(18%) had low levels.
In our study, the immuno expression of Ki-67 was seen in all 50 cases of OSCC. The relationship of Ki-67 expression was statistically significant when compared with histological grading (p value=0. 0121).
On a study of 35 patients with intraepithelial lesions and SCC, Intisar Salim et al([44]), observed an increase in over expression of Ki-67 with increase in grade of histopathologic abnormalities in oral IEL and SCC. Ki-67 LI of highest level belonged to poorly differentiated SCC (87. 7%) and high grades IEL (80%).
Rakheja Mahima et al (2015)[45]in 105 cases showed closely similar results. A highly significant correlation was found between the expression of Ki-67 and grades of OSCC (p<0>
Abhay R. Chandak et al (2011)[9]studied that Ki-67 LI showed a significant increase from well differentiated to moderately differentiated OSCC.
A significant correlation was observed by Carlos de Vicente et al (2002)[46] observed between the histological grading and Ki-67 labelling index.
Kannan et al (1996),[47] and Premlatha et al (2010)[48] observed that on statistical analysis (with chi-square test), a highly significant correlation was observed between the advancing grades of oral squamous cell carcinoma and expression of Ki-67. (p<0>
According to Kurokawa et al[49]Ki-67 over expression on tumour borders, is associated to its histological grade (p<0>[50] did not find any significant correlation of Ki-67 positivity when compared with histological differentiation.
While Piattelli et al (2002)[51] could not show significant differences among different grades of OSCC.
Correlation of Ki-67 Expression and “TNM” staging
Our study did not show significant correlation of Ki-67 expression with TNM staging.
This is in accordance with study done by A. R. Chandak et al (2011)[9] on 50 cases of oral squamous cell carcinoma whose results also showed no significant correlation of Ki-67 LI with TNM staging.
Conclusion
Oral squamous cell carcinoma is significantly associated with elderly age group and with male sex. Commonest site being buccal mucosa and is most commonly associated with combined habit of smoking and tobacco chewing.Higher expression of Ki-67 was seen which was statistically significant when compared with histological differentiation. This highlights the importance of Ki-67 in squamous cell carcinoma. In the histopathological assessment Ki-67 helps supplement the clinical data.
Hence we propose that Ki-67 is a useful marker of proliferation. It can predict the transition of oral epithelial dysplasia to malignancy.
Conflict of Interest: None.
Doi No:-10.18231/2581-3706.2019.0001