Introduction
HBV infection is one of the world's most serious health problems. worldwide 350 million people are living with chronic HBV infection.1 Takai S et al showed that reactivation of HBV is more common in hemato-oncological patients (3.26 to 22%) than in the general population.2, 3, 4, 5 Dervite I et al suggested that Chemotherapy for both solid and haematological cancers has been reported to cause reactivation, particularly when rituximab is used. 6 Chemotherapy-induced reactivation can happen during or after treatment. HBV reactivation can delay chemotherapy and lead to fulminant liver failure and mortality. Lucifora J et al shows Even though HBV infection has been completely cured in affected persons, the viral genomic structure is still present in the hepatocyte nucleus of all infected individuals.7 In cancer patients with previously occult or cured HBV infection, HBV reactivation is common because of immune suppression. Lok AS et al reported Hepatitis-like clinical and biochemical symptoms, as well as an increase in HBV DNA, characterize HBV reactivation.8
HBsAg negative, HBcAb positive individuals have a lower chance of reactivation of the hepatitis B virus than HBsAg positive patients while undergoing chemotherapy. It's ranging from 5% in Western nations to more than 50% in Eastern countries.9, 10, 11 But Limited data in our countries.
All the most recent hepatitis B recommendations, including EASL 2017,12 AASLD 2018,13 and INASL 2018,14 recommend screening before initiating chemotherapy in all cancer patients. Clinical oncology recommendations (ASCO) are ambiguous, and they do not recommend regular HBV infection screening in all patients.15
In patients taking systemic chemotherapy and who have HBsAg positive, the risk of HBV reactivation is very significant. Therefore, the current guidelines recommend antiviral prophylaxis for these patients. However, there is a lack of evidence on managing HBV reactivation in HBsAg-negative and anti-HBc-positive people on chemotherapy.
The purpose of this study was to assess how often patients with HBV infection that had been resolved (HBsAg negative, anti HBcAb positive) reactivated the virus while undergoing chemotherapy for various cancer.
Materials and Methods
This prospective descriptive analysis included all patients with various tumors who had or were undergoing chemotherapy and had evidence of HB reactivation (HBVR) from March to October 2021. All of these individuals were HBsAg negative and HBcAb positive before commencing treatment. The research was carried out at Tirunelveli Medical College's Department of Medical Gastroenterology. The local institutional ethics committee gave its approval to this study.
Hwang JP et al reported Transaminitis -(ALT > 3 times the upper limit of normal and >10 fold rise in HBV DNA levels from baseline levels or detection of HBV DNA with a level > 100000 IU/ml in a person who had no baseline
HBV DNA) and HBsAg serocon version from negative to positive is characterized as HBV reactivation. 16
Inclusion criteria
This study included all cancer patients who were HBsAg negative but HBc Ab positive and were receiving chemotherapy.
Exclusion criteria
Patients with hepatitis B, either acute or chronic, did not meet the HBVR criteria.
Patients who have been infected with multiple hepatotropic viruses.
Patients with HCC.
Patients who are pregnant or nursing their babies.
Before beginning the trial, the patients signed a written informed permission form. we did a thorough examination and extensive history (drug history, kind of cancer, risk factors, and surgery) for all patients. Complete blood count, liver function test, coagulation parameters, and abdominal ultrasonography were all provided as part of the baseline study. HBsAg was detected using the ELISA technique. The total and IgM levels of anti-HBc antibodies were determined using an enzyme polymerase chain reaction method immunoassay. Hepatitis B viral DNA was measured in the patients' plasma using a real-time PCR method
Results
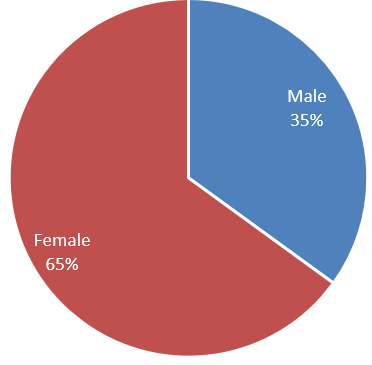
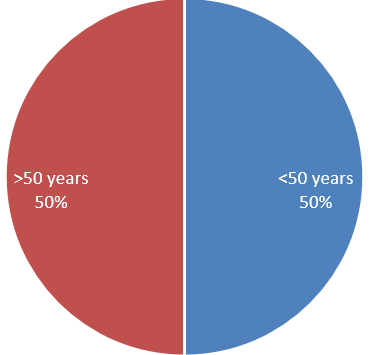
Data on 40 patients with various cancers who were undergoing chemotherapy showed signs of HBV reactivation in previously HBsAg negative and HBcAb positive patients. Among 40 cohorts, 23 (65%) patients were female and 13 (35%) patients were male patients. In all patients, 50% were >50years and 50% were <50years but all above 40years. All patients had HBsAg positive and total plus IgM HBc Ab positive. All cohorts show evidence of HBVR in the form of ALT >3 times upper limit and HBV DNA level >1,00,000 IU/ml in 70% and >20,000 IU/ml in 30%.(Figure 1, Figure 2)
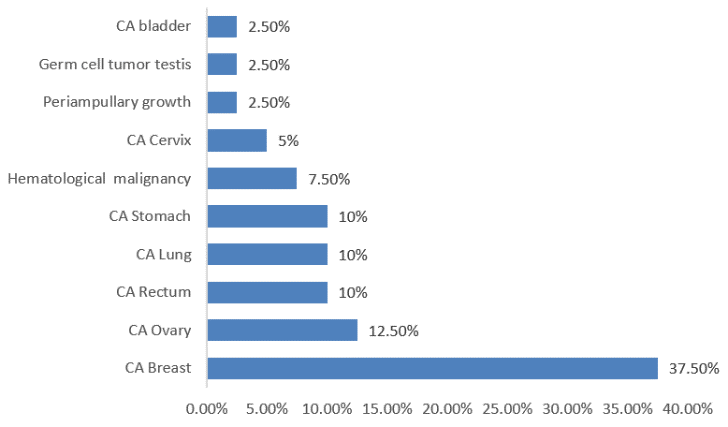
Among 40 cohorts 15 patients (37.5%) were suffering from CA breast, 5 patients (12.5%) had CA ovary, 4 patients (10%) had CA rectum, 4 patients (10%) had CA lung, 4 patients (10%) had CA stomach , 2 patients had CA cervix(5%), 3 patients (7.5%) had hematological malignancy, 1patient (2.5%) had periampullary growth, 1 patients (2.5%) had bladder cancer and 1 patients (2.5%) had germ cell tumor testis.(Figure 3)
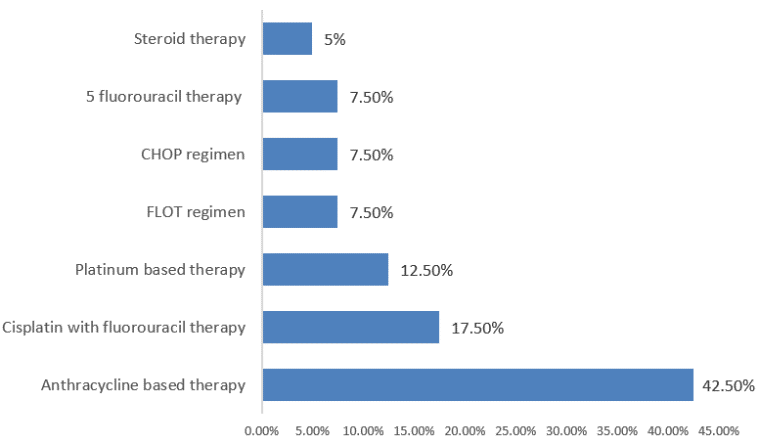
Among 40 cohorts, 17 patients (42.50%)received anthracycline-based therapy, 7 (17.5%)patients received cisplatin plus fluorouracil therapy, 7 (12.5%)patients received platinum-based therapy, 3(7.5%)patients had received FLOT regimen, 3 (7.5%)patients received CHOP regimen, 3 (7.5%) patients 5 fluorouracil therapy and 2 (5%) patients had received steroid therapy.(Figure 4)
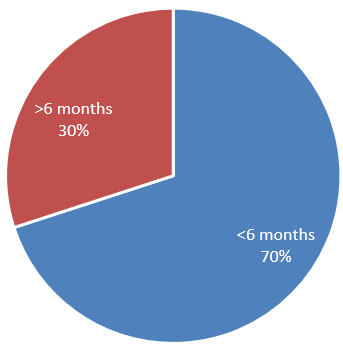
Among 40 patients, 28 (70%) patients had reactivation within 6 months of chemotherapy and 12 (30%) patients had reactivation after 6 months of starting chemotherapy.(Figure 5)
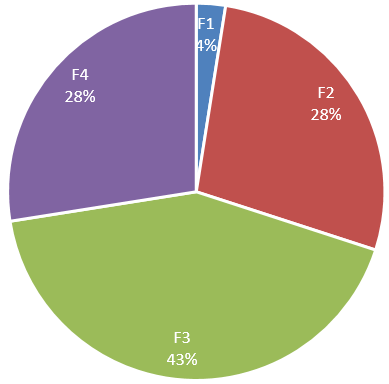
Among 40 patients 11 (27.5%) patients F4 fibrosis and 11 (27.5%) had F2 fibrosis, 17 (42.5%) had F3 fibrosis and 2 (2.5%) patients had F1 fibrosis.(Figure 6)
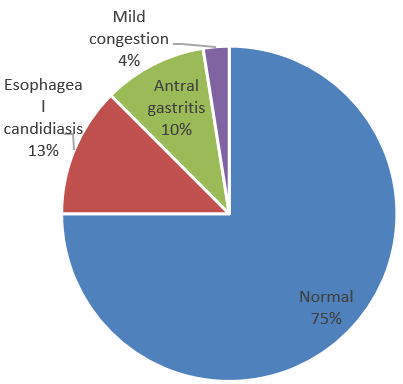
75% had normal findings, 12.5% had esophageal candidiasis, 10% had antral gastritis and 2.5% had mild congestive changes.(Figure 7)
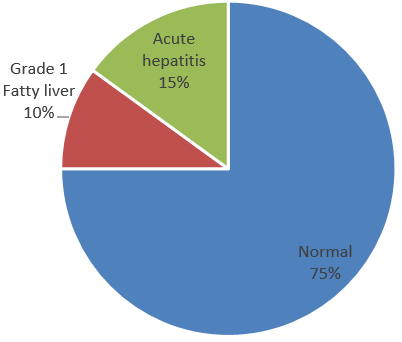
30 (75%) patients showed normal study, 4(10%) patients had grade 1 fatty liver, and 6 (15%) patients had acute hepatitis.(Figure 8)
Discussion
Due to increased exposure to blood products, surgeries, and, most importantly, virus reactivation, hepatitis B infection is more common in cancer patients than in the generalpopulation.4 Wani et al showed hematological malignancies have four times the rate of HBV reactivation as compared to solid.17 Kim et al.18 found that one patient out of 321 with solid tumours who were HBsAg negative and HBcAb positive had HBV reactivation, whereas 12 out of 365 patients with haematological malignancy had HBV reactivation.15 Our study included 37 patients with solid tumors and all of them had HBV reactivation. Most individuals in our study had solid tumors rather than haematological malignancies.
Wani et al. showed that most patients with HBV reactivation were treated with polychemotherapy, which included anthracycline-based therapy, anthracycline plus steroid and rituximab-based therapy.16 Other chemotherapies include fluorouracil-based therapy, CHOP regimen, FLOT regimen, platinum-based, 5 FU, and steroid therapy. Our study also shows similar data. No one received rituximab-based therapy even though it is the most common drug to induce reactivation.
Several studies have confirmed that HBV reactivation following chemotherapy and the median interval between the initiation of chemotherapy and onset of reactivation is 4 months (range 1-9 months).3, 8, 19, 17, 20, 21, 22, 23 In our study (70%) experienced reactivation within 6 months of starting chemotherapy, while 30% experienced reactivation after 6 months. Almost all patient shows acute hepatitis pattern in the form of raised ALT level >10 times and very high HBV DNA level. According to most prior studies, reactivation is more common among HBsAg positive individuals following chemotherapy for various malignancies. HBV reactivation is well documented in individuals receiving bone marrow transplantation and treatment for haematological malignancies with HBsAg negative and anti-HBc positive serology.24, 25 But data regarding HBV reactivation in patients with HBsAg negative and HBcAb positive patients undergoing chemotherapy for solid tumor sparse.
S.Fidan et al. show that HBV reactivation was seen in resolved HBV infection receiving chemotherapy for solid tumors. 26 Our study demonstrates also similar results that are HBsAg-negative and HBcAb-positive individuals undergoing chemotherapy for solid tumors likely to reactivate. Therefore, all the recommendations suggest that patients with HBV reactivation who are receiving cancer chemotherapy and are HBsAg positive must begin antiviral prophylaxis one week before the chemotherapy.
In HBsAg negative and anti-HBc positive haematological malignancy patients on a rituximab-based regimen and stem cell transplantation, anti-HBV prophylaxis is recommended, regardless of their baseline blood HBV DNA level. However, the prevalence of HBV reactivation and the necessity for antiviral prophylaxis in patients receiving chemotherapy for cancers other than haematological malignancies remains unknown.
Conclusion
According to our findings, HBV reactivation was also reported in HBsAg negative and HBcAb positive persons following chemotherapy for solid tumors. As a result, all cancer patients receiving chemotherapy should have their HBV infection evaluated, including HBsAg, anti-HBc, and anti-HBsAb titer levels. In addition, patients should be regularly monitored for HBV DNA positive and HBsAg seroconversion for one to three months, and rescue antiviral medication should be initiated as soon as HBV reactivation occurs because reactivation in these patients can be lethal. Regardless of HBV DNA or HBsAg seroconversion, antivirals may be started for HBsAg negative and HBc Ab positive patients with solid tumours who are undergoing chemotherapy. But it needs further study.