Introduction
Before the COVID-19 pandemic, Tuberculosis (TB) caused more deaths than any other single infectious agent, including HIV/AIDS.1 As of 2021, the number of TB patients notified has increased by 19% compared to 2020. 2
Tuberculosis (TB) is an ancient bacterial disease affecting humans, caused by Mycobacterium tuberculosis. It manifests in two main types: pulmonary TB (PTB) and extrapulmonary TB (EPTB). Extrapulmonary TB occurs when the infection affects organs outside the lungs, with the lymph nodes being the most frequently affected site. 3 EPTB cases constitute approximately 15-20% of all TB cases. 4
The most common manifestation of mycobacterial infections is cervical lymphadenitis, 5 and TB of the head and neck can be difficult to diagnose due to a large number of smear-negative cases. This often leads to positive cases being missed, which increases the burden of TB. 6
Conventional methods for diagnosing EPTB include fine-needle aspiration cytology of lymph nodes and demonstrating acid-fast bacilli (AFB) using Ziehl-Neelson (ZN) staining. 7 Fine-needle aspiration cytology of lymph nodes can be presumptively diagnosed with tuberculous lymphadenitis morphologically. 8 The cartridge-based nucleic acid amplification test (CBNAAT) is a real-time polymerase chain reaction (PCR) assay designed to swiftly detect the presence of Mycobacterium tuberculosis and rifampicin resistance in biological samples, providing results within a two-hour timeframe. Acknowledged by the World Health Organization (WHO) in December 2010, this technology stands as a pivotal advancement in global tuberculosis (TB) control efforts. 9
Materials and Methods
The prospective study was done at DR B. R Ambedkar Medical College & Hospital, Bangalore from October 2021 to January 2023. The study consists of clinically suspected cervical tubercular lymphadenopathy patients referred from various departments of this hospital for FNAC.
Inclusion criteria
Patients aged above 18 years and below 65 years who are clinically suspected with cervical tubercular lymphadenopathy.
Study procedure
Prior to conducting Fine-Needle Aspiration Cytology (FNAC), the patients were provided with an informed consent form in the language of their choice. Their clinical history and relevant investigations were thoroughly reviewed. During the Fine Needle Aspiration Cytology (FNAC) procedure, a 22-gauge needle, coupled with a 10 ml syringe, was employed to ensure the collection of sufficient and representative samples. Adequate smears were made and immediately fixed and prepared for cytomorphological analysis using Hematoxylin and Eosin (H&E) and Papanicolaou stain (PAP). One smear was air-dried and stained for acid-fast bacilli while the remaining aspirate was rinsed into a Falcon tube with 5 ml of normal saline and processed for CB-NAAT testing. The procedures were executed in adherence to the operator manual specifications outlined by the Central TB Division, Government of India, for the Gene Xpert system. The assay sample reagent, comprising a mixture of sodium hydroxide and isopropanol in a 2:1 ratio, was meticulously introduced to the sample. Following a 15-minute incubation period at room temperature with periodic agitation, 3ml of the processed sample was meticulously loaded into the cartridge and seamlessly integrated into the CB-NAAT instrument module. The subsequent assay steps and conclusive results were automatically presented on the Gene Xpert monitor approximately 1 hour and 50 minutes after initiation.10
Results
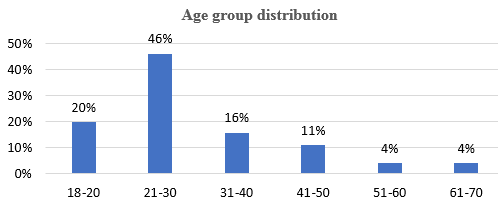
A total of 102 cases underwent FNAC based on clinical and radiological findings. Females were the majority (Table 1) and the most common age group was 21-30 [Figure 1].
Upon examination, all cases showed lymph node enlargement. Of these, 63 cases were discrete, and 39 were matted. Most cases were found in level V, as shown in [Table 2].
Table 2
Distribution of level of involvement of lymph nodes.
|
Level of lymph node |
No. of cases |
|
I |
13 |
|
II |
5 |
|
III |
14 |
|
IV |
26 |
|
V |
44 |
During aspiration, most cases showed blood mixed with aspirate. The CBNAAT and AFB tests were more positive in the caseous aspirate. [Table 3]
Table 3
Types of aspirates with CBNAAT and AFB positivity.
|
Types of aspirates |
Number |
No of CBNAAT positive cases |
No AFB-positive cases |
|
Blood mixed |
65 |
23 |
6 |
|
Purulent |
29 |
24 |
8 |
|
Caseous |
8 |
7 |
5 |
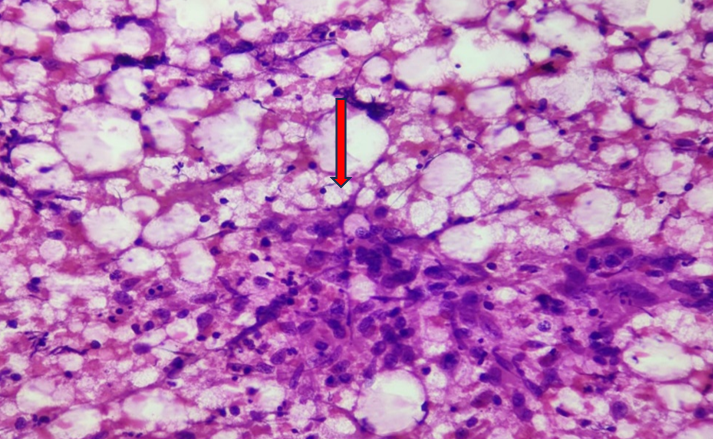
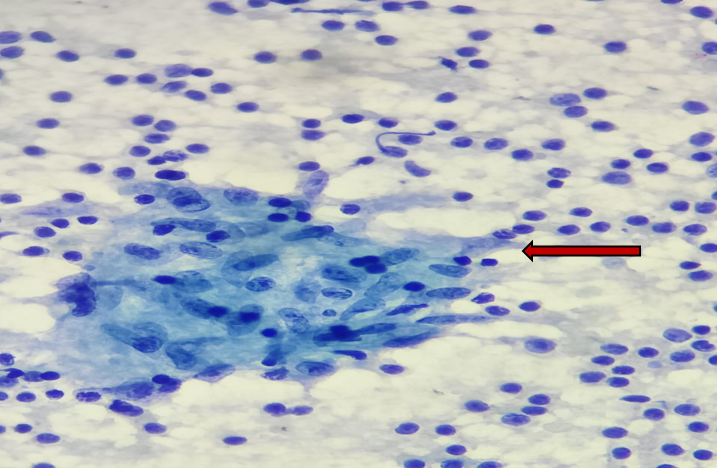
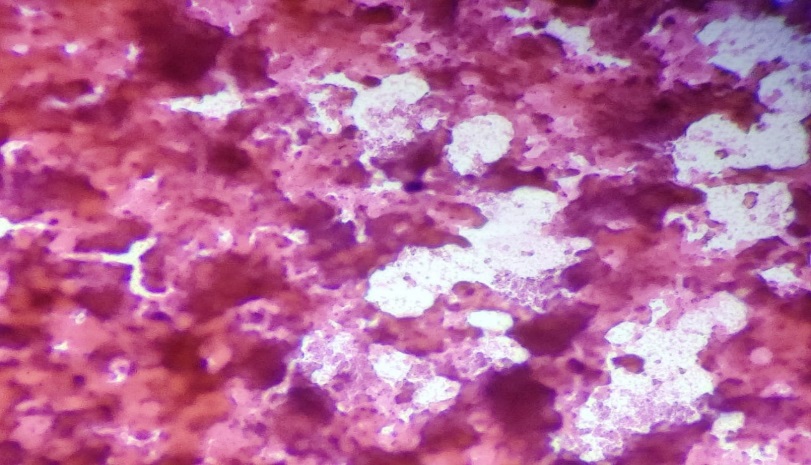
Among cytomorphological findings of 102 cases [Table 4], 46 cases showed granulomatous lymphadenitis with necrosis [Figure 2],12 cases showed granulomatous lymphadenitis without necrosis [Figure 3] and 3 cases showed necrotizing lymphadenitis [Figure 4].
Table 4
Cytomorphological findings with CBNAAT and AFB positivity.
In this study, among 102 cases 54 cases showed CBNAAT positive, and 19 cases showed AFB positive [Figure 5].
Figure 5
FNAC findings show the presence of AFB against a necrotic background in ZN staining. (Oil immersion,1000X)
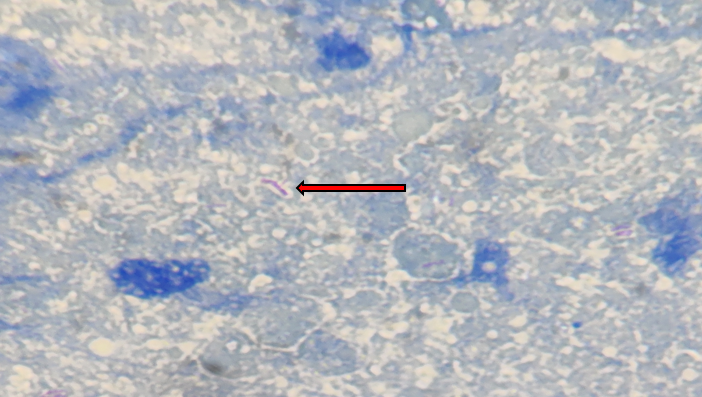
Table 5
Compares age group and sex preponderance with the other studies.
|
Study |
Period |
Age group |
% of cases |
Females |
|
Present study |
Oct 2021 – Jan 2023 |
21-30 |
46% |
58.82% |
|
Mulualem et al |
May-Sept 2013 |
16-30 |
58% |
53.1% |
|
Bryan Rock et al11 |
Jan1993-Dec 2003 |
15-24 |
43% |
54% |
|
Adhikary et al12 |
Mar 2021- Feb 2022 |
21 - 30 |
27.85 % |
59.49 % |
Table 6
Compares the sensitivity and specificity of the present study with the other studies.
|
Study |
Study Period |
Sensitivity |
Specificity |
|
Present study |
October 2021-January 2023 |
81.97% |
90.2% |
|
Arpitha et al13 |
July 2019- June 2020 |
80% |
86% |
|
Suwarna B. Patil et al14 |
January 2019 - December 2019 |
55.5 % |
83.80% |
|
Lavanya et al15 |
April 2018 to March 2019 |
28.75% |
88.7% |
|
Komanapalli SK et al16 |
April 2017- March 2018 |
84.25 % |
86.71% |
In total, CBNAAT exhibited positivity in 54 cases, while AFB demonstrated positivity in 19 cases. In comparison to FNAC, CBNAAT displayed a sensitivity of 81.97%, a specificity of 90.2%, a positive predictive value of 92.6%, and a negative predictive value of 77.1%. In contrast, AFB exhibited a sensitivity of 31.15%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 49.4%.
Discussion
This prospective study, conducted at a tertiary care hospital, aimed to diagnose cervical tubercular lymphadenitis by comparing FNAC with AFB staining and CBNAAT. FNAC, recognized as the primary diagnostic test, holds significance in detecting tubercular lymphadenitis.17
In this study, we have included only cervical lymph nodes as it is the most common site of EPTB.3
This present study shows the greatest number of cases affected in the 21-30 age group with female preponderance. This is similar to studies conducted by Mulualem et al, Bryan Rock et al and Adhikary et al18, 11, 12 [Table 5].
All 102 cases showed lymph node enlargement and the highest number of patients presented with discrete swelling (61.76 %) and the majority of the lymph nodes were seen in level V (43.14 %). These findings are comparable with the study done by Sreenidhi et al. 19
While analyzing the types of FNAC aspirates, CBNAAT showed the most (87.5%) positivity in caseous aspirate and the least (35.38%) in hemorrhagic aspirate. This is similar to the study conducted by Tadesse M et al which shows the most (69%) positivity in caseous aspirate and the least (41.7 %) in hemorrhagic aspirate.18 Hence CBNAAT is less sensitive to blood-mixed samples.
In the study conducted by Khare et al 2014, smears from lesions having only caseous necrosis showed maximum AFB positivity (94.1%) which is comparable with our study which also showed maximum AFB positivity (62.5%) in caseous aspirate. 20
The presence of clusters of epithelioid cells, with or without giant cells and/ or caseous necrosis is the diagnostic criteria for tuberculosis in FNAC. 21 This study shows 100% CBNAAT positivity in necrotizing lymphadenitis which is similar to the findings in the study done by Kalyani Gouda et al 22 and Anjali R et al. 23
Out of 102 cases, FNAC is positive in 61 cases and confirmed in 19 cases by AFB and 50 cases by CBNAAT. Among 41 FNAC-negative cases, CBNAAT could detect Tb in 3.9 % of cases. Thus, CBNAAT showed its importance in finding out missed cases among FNAC and AFB negative cases.
This present study showed sensitivity of CBNAAT is 81.97% which is more than that of AFB (31.15%). Similar findings are also noted in studies conducted by Lavanya et al and Suwarna B. Patil et al. 15, 14
This study also showed specificity of CBNAAT is more than that of its sensitivity. These are comparable with the studies conducted by Arpitha K et al, Lavanya et al Suwarna B. Patil et al and Komanapalli SK et al13, 15, 14, 16 [Table 6].
Conclusion
According to this study, CBNAAT is more effective than AFB in detecting EPTB in FNAC missed cases. Moreover, CBNAAT is more sensitive than AFB. While culture is considered the gold standard test for EPTB diagnosis, CBNAAT has a faster turnaround time. Therefore, in countries like India, where the burden of TB is high, CBNAAT can be used as a rapid diagnostic test to prevent underdiagnosis and provide early treatment.