Introduction
In later half of twentieth century the most common medical disorder which was being described was sleep apnea or sleep hypoapnea syndrome. During sleep partial or total collapse of upper airway is a characteristic finding in patients of Obstructive sleep apnoea/hypopnoea syndrome (OSAHS).1 The two most important consequences of OSA are recurrent nocturnal hypoxemia and sleep fragmentation; the latter results in a marked reduction in time spent in stages 3 and 4 sleep.2 Both of these have important metabolic consequences and hormonal abnormalities.3 Sex hormones (testosterone, estrogen/progesterone) secretion rises as sleep begins and continues to increase throughout the night. Patient of Obstructive sleep apnoea due to disturbed sleep may fail to produce sex hormones that leads to diminished libidio and impotence.
Increased progesterone in females during pregnancy may protect the upper airway patency. But it has been suggested that increase estrogen level cause edema and mucus production in the upper airway tract that leads to breathing disturbance.4
There was a reduced response to hypoxia when the progesterone receptors were knocked down in mice by Potvin et al in their study.5 In a study done by Driver et al they found that females have a lower upper airway resistance during luteal phase of menstrual cycle than follicular phase.6
Females are thought to be protected due to hormones from sleep disordered breathing upto their menopause.7
In sleep clinics it has been seen by Dancey et al that among the female patients 47% were postmenopausal and only 21% were premenopausal.8
Various other studies have also shown that prevalence of sleep apnea disorder increases with age and menopause in females.9, 10, 11, 12
Estrogen therapy among post menopausal females have been found to decrease the incidence of sleep disordered breathing and the effect of estrogen-progesterone combined therapy in post menopausal females has a further synergistic effect on sleep breathing disorders. 4 Recently it has been seen that females with polycystic ovary disease have higher prevalence of sleep disordered breathing. 13, 14, 15
In obstructive sleep apnea syndrome patients who are obese have lower testosterone levels and those who are massively obese have decreased levels of free testosterone as well. 16, 17
Sleep apnea causes sleep deprivation which alongwith prolonged stress and sleep fragmentation leads to a fall in testosterone levels. 18, 19
Circadian rhythm of secretion of testosterone is also affected due to repeated episodes of hypoxaemiain patients of obstructive sleep apnea syndrome. Luteinizing hormone levels are also affected due to hypoxia. 20, 21, 22
Greater the severity of hypoxaemia lower will be the level of testosterone in patients of sleep apnea. 20, 21, 22 Witteret et al found that with proper oxygen therapy and weight reduction in patients of obesity hypoventilation syndrome testosterone levels were found to increase. 23
Testosterone is secreted in a cyclic manner, mostly due to sleep patterns, and not circadian rhythm. Its concentration peaks in the blood during sleep in the first REM phase and reaches its lowest level in the afternoon. Three hours of sleep with normal architecture should be sufficient to maintain testosterone secretion. Additionally, testosterone is subordinated to LH pulses which occur every 90 minutes. 24
Important known risk factors for sleep disordered breathing are male gender and obesity. 25
Obsese males have poor quality of sexual life as thay have less reproductive potential which is due to lower total and free testosterone levels. 26, 27, 28
In a study done by Jauch Chara et al in healthy individuals they found that sleep deprived individuals had significantly lower levels of total testosterone and prolactin hormone. 29
Further larger studies are required to give more clarification about independent effect of sleep apnea in both males and females affecting their hormonal levels.
This study was done to see the relation between sleep apnea and sex hormone like testosterone, estrogen, progesterone, LH and FSH levels, and sexual quality of life in severe OSA patients. An adequate understanding of this relation, independent of weight, can help the conceptualization and validation of the results of studies that would look at the effect of correction of sleep apnea on serum testosterone and sexual function in men
Materials and Methods
This was a case control cross-sectional study, prospective in nature carried out in the department of Respiratory Medicine, Jawaharlal Nehru Medical College, AMU, from June 2018 – December 2020. Institutional ethical clearance was obtained for the same.
Hormonal analysis
Early morning around 10 ml venous blood sample was drawn from all participants after 12 h of overnight fasting. In premenopausal females blood sample was drawn in early phase of menstrual cycle because of low variability of sex hormones in follicular phase. The following were measured: total testosterone (in males), estrogen and progesterone (in females), LH and FSH (in both males and females) using electrochemiluminescent immunoassay. Refer to table no. 1 for normal reference range of hormones taken for the study.
Sleep studies
Participants were subjected to sleep assessment in ward in Level I sleep lab. The system used was Philips Alice 6 LDe with 32 leads.
Study initiated between 9:30-10:00 PM and terminated between 5:30-6:00 AM. At the time of recording weight and gender of participants was unknown.
The parameters recorded were as follows:
Snoring, ECG, pulse transit time, airflow, nasal pressure, respiratory efforts, position of body and oxygen saturation level (SaO2).
A reduction of Thirty percent or more from baseline in airflow which lasted for at least 10 seconds or fall in oxygen saturation level by more than or equal to four percent from the baseline was defined as hypoapnea.
Cessation of airflow for more than 10 seconds was defined as apnea.
Apnea can be central or obstructive. Central apneas are characyerised by absence of airflow and no respiratory effort while obstructive apneas are associated with increased pulse transit time and increased respiratory efforts.
The ratio of the number of episodes of apnea or hypopnea per hour of reported sleep time is the apnea-hypopnea index (AHI) and American Academy of Sleep Medicine (37) has classified OSA into three categories on the basis of AHI as follows:
Mild OSA: AHI > 5 and < 15
Moderate OSA: AHI ≥ 15 and < 30
Severe OSA: AHI ≥ 30.
Statistical analysis
SPSS version 25.0 was used for data analysis. All data like demographic, anthropometric, hormonal (LH, FSH, Estradiol, Progesterone, and testosterone) was presented as mean and SD with 95% and the significance of relation between obstructive sleep apnea and various hormonal disturbances made through ANOVA statistical method. P score of less than 0.05 was taken as statistically significant and p score of less than 0.01 was taken as statistically highly significant. . Pearson correlation coefficient was calculated between AHI and all hormones separately.
Results
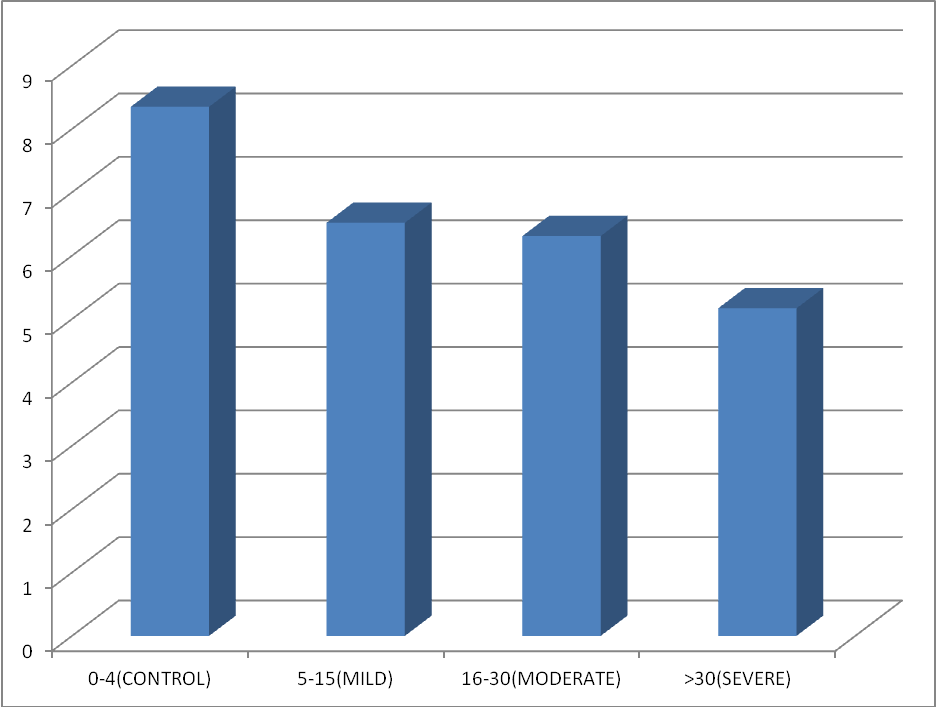
Out of 125 subjects who underwent polysomnography test, 65 subjects with mean age (50.2 ± 7.12) years, mean weight (63.5 ± 7.3) kgs, mean height (158 ± 6.8) cms and mean BMI (38.65 ± 4.55) kg/m2 were taken as cases whose AHI was more than 5. 60 non OSA subjects with AHI < 5 matched for Age, gender and bodymass index were taken as controls.. This study included 42 males and 23 females, out of which 9 were pre-menopausal and 14 were post-menopausal.
The distribution of the cases was as follows:
hows the distribution ofanthropometric and hormonal profile according to severity of OSA. The association between severity of OSA and weight of patients and BMI was found to be statistically significant using ANNOVA test (p < 0.05).
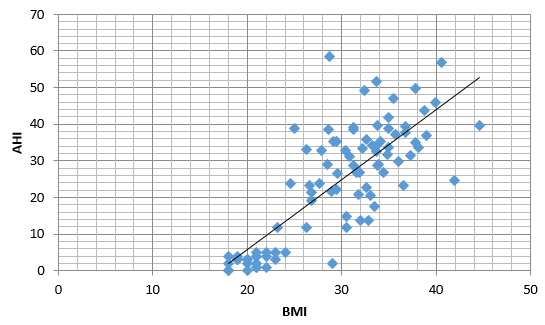
Figure 1 shows a positive and statistically significant correlation between subjects BMI and AHI using Pearson’s correlation test (r= 0.79, p= <0.001).
Mean AHI calculated in different classes of obesity in study population was as follows: (21.34 ± 8.23)/h in pre-obesity group, (21.34 ± 8.23)/h in obesity class I, (27.14 ± 9.38)/h in obesity class II, (31.7 ± 8.16)/h in obesity class III and (40.86 ± 13.11)/h in obesity class IV. A clear rising trend was observed in AHI with increasing severity of obesity. (F3 86=8.3, p<0.001).
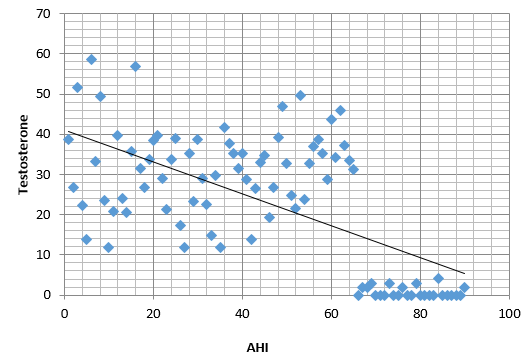
In this study the mean value of testosterone lied on the lower side of the normal reference range amongst sleep apnea subjects and an decreasing trend was observed in mean testosterone levels with increasing severity of OSA (Table 2 and Figure 2). Data was statistically significant (F386=5.26, p < 0.01). Figure 3 shows a scatter dot plot between subjects AHI and total testosterone levels which was statistically significant (Pearson’s correlation coefficient r = −0.41, P = 0.001)
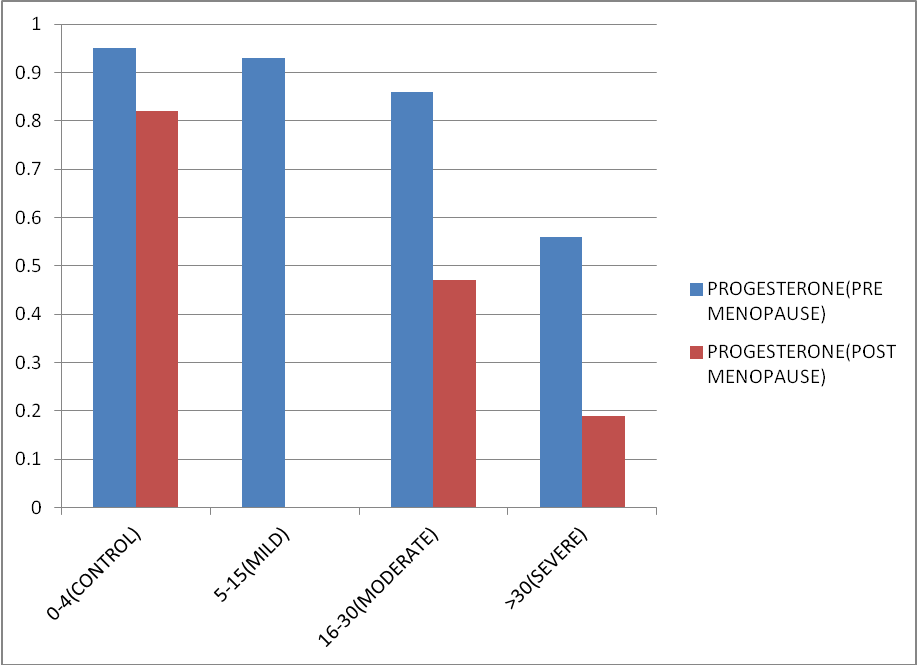
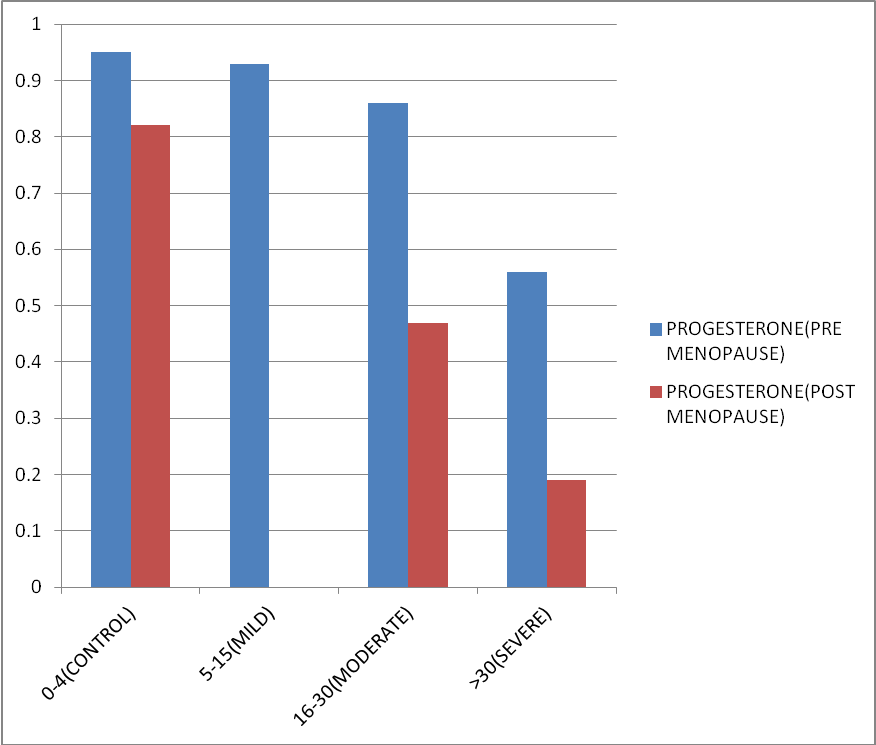
The mean value of estradiol and progesterone in both pre-menopausal and post-menopausal females in all the groups of OSA also lied on the lower side of normal range. Mean value of both the hormones decreases with increasing severity of OSA. Figure 4, Figure 5 represents the same. Data was statistically significant as p-Value is <0.01. (F386=10.17, p < 0.01).
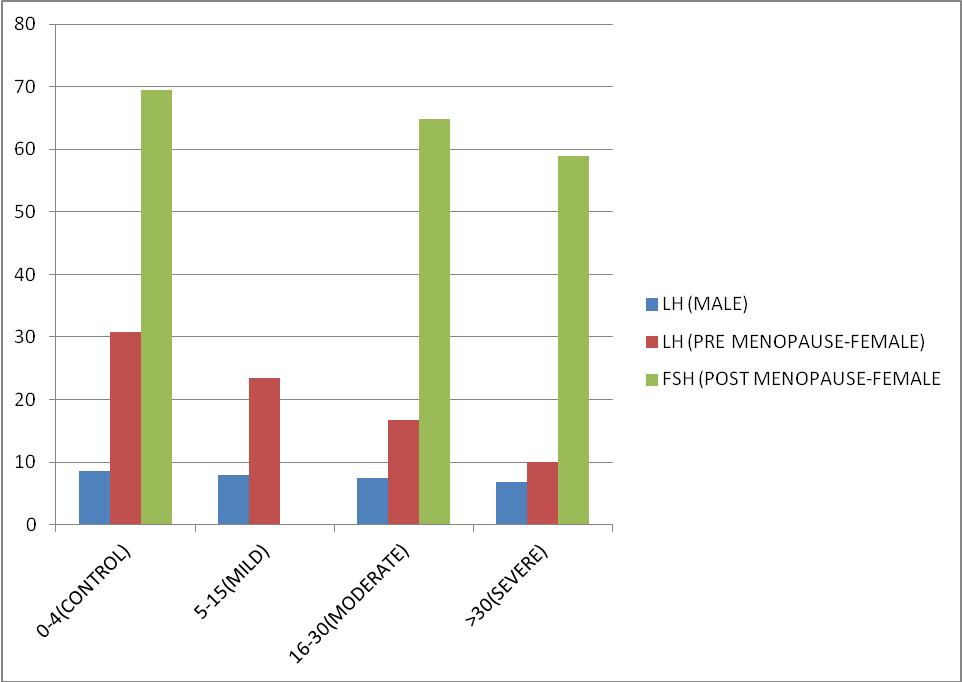
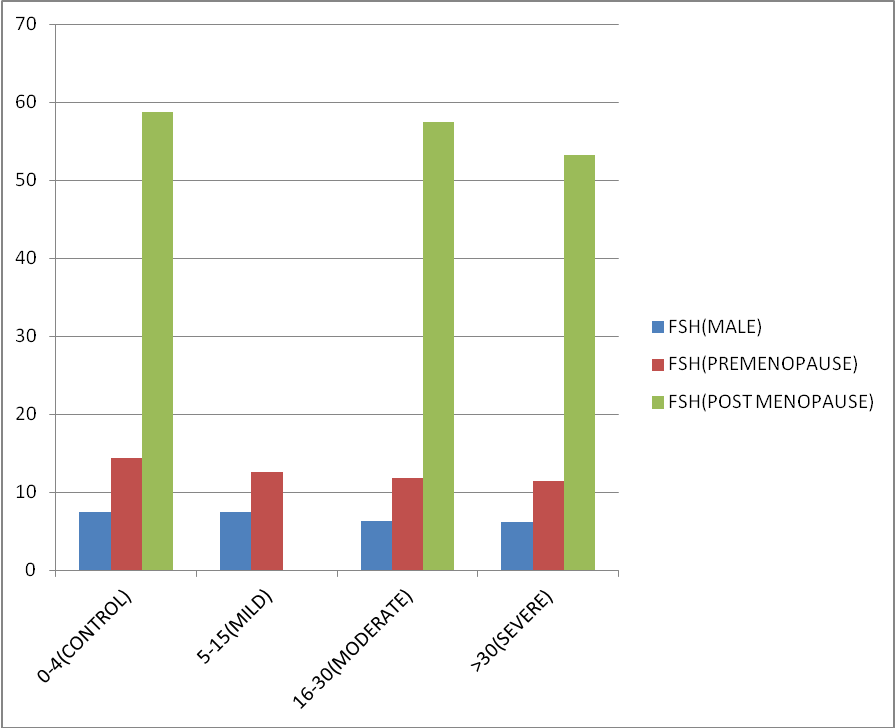
Similarly on comparing mean LH and FSH of males, pre-menopausal female and post-menopausal female in different categories of OSA according to severity (Table 2), a decreasing trend was observed with lowest mean value of both the hormones in severe OSA group (Figure 6, Figure 7). Although data was not statistically significant as p value is > 0.05.
Table 1
Normal reference range of sex hormones in males and females taken in study.
Table 2
Comparing means of anthropometric and hormonal profile of study population between different classes of severity of OSA.
Discussion
Obstructive sleep apnea (OSA) is a serious health disorder which contributes to cardiovascular, metabolic and hormonal complications, decreased work productivity, loss of libido, depression, automobile accidents, and even death. In our study out of 23 female diagnosed as OSA 14 (61%) were postmenopausal and 9 (39%) were premenopausal and on applying chi- square test the difference in the prevalence of premenopausal and postmenopausal female were highly significant with p value of <0.05. Edward O. Bixler et al 9 did one night PSG study on 1000 female patients and found same results. In this study females were divided into three categories: (1) Premenopausal females; (2) Postmenopausal females without hormone replacement therapy (3) postmenopausal females with hormone replacement therapy.
Prevalence of OSA in premenopausal females was 0.6% as against 1.9% in post menopausal females (RR=0.3 [0.1, 1.2], p=0.04). Among postmenopausal females those who were taking hormone replacement therapy had prevalence of OSA similar to premonopausal females (0.5 versus 0.6%) (RR=0.8 [0.2, 7.9], p=0.91), and less than postmenopausal females not taking hormone replacement therapy (0.5 versus 2.7%)(RR=0.2 [0.2, 1.7], p=0.0 4) . While prevalence in postmenopausal females not taking hormone replacement therapy was higherthan premenopausal females (2.7 versus 0.6%)(RR=4.7 [1.2, 14.5], p=0.02) and was more similar to the prevalence of OSA in men (2.7 versus 3.9%) (RR=0.7 [0.4, 1.5], p=0.39).
Ulla Anttalainen et al. 30 studied in 601 women between 1994 and 1998 and found that the prevalence of SDB is higher in postmenopausal females than in premenopausal females(86.2% versus 79.4%, p =0.057).
From the above study it is clear that OSA is common in postmenopausal female as compared to premenopausal female because estrogen and progesterone have a role in maintaining the upper air way patency and thus protect from developing OSA. In postmenopausal female level of estrogen and progesterone decreases, therefore increasing risk of developing OSA. 30 They observed that postmenopausal female with HRT had less prevalence of OSA in comparison to postmenopausal female without HRT.
In our study we have found that as the BMI increases, the severity of OSA increases as well. Peppard PE et al studied 690 patients selected randomly over a period of four years and concluded that a ten percent increase in body weight is associated with approximately thirty two percent increase in apnea hypoapnea index (95% confidence interval [CI], 20%-45%) and a ten percent reduction in body is predicted to decrease apnea hypoapnea index by around twenty six percent (95% CI, 18%-34%). 31 The odds of developing moderate to severe SDB increases by around six times by a ten percent increase in body weight (95% CI, 2.2-17.0). Kuo-Tung Huang et al.32 also found similar results.
Fragmentated sleep and hypoxia in obese individuals may lead to central suppression of testosterone.
In our study we have studied in 65 OSA diagnosed patients and compare with 60 healthy control with matched BMI and age as cases .We found lower testosterone in cases in comparison to control (p-value < 0.01) with lowest level of testosterone in severe OSA because there is a negative correlation between AHI and testosterone ( r = -0.4). Similar trend was observed for LH and FSH but was statistically not significant (r was equal to -0.09, p- value was more than 0.05). Our result is allied to the study of J. D. Santamaria et al. 33 In their study Lower serum testosterone (T) levels were documented in 15 men with OSA versus nine snorers (no OSA), (9.18 ± 0.92 vs 11.55 ±0.90 nmol/l, mean ± SEM), P < 0.05 and prospective controlled trial of uvulopalatopharyngo-plasty therapy (UPP) for OSA in 12 subsequent subjects showed reproductive improvement which was parallel with improved apnea at 3 months post-surgery. T increased (13.31 ± 1.07 to 16.59 ± 0.72 nmol/l), P<0.05.in their study it was also observered by them that on follow up of patients after three months of uvulopalatopharyngoplasty patients reported improvement in their libido and sexual functioning. Fernando Drimel Molina et al. 34 also found similar result when he studied in 103 diagnosed OSA patients and among them 24% had lower serum testosterone level with p-value < 0.05. In an extensive cross-sectional study of 225 men undergoing polysomnography by Grunstein RR et al.35 the reduction of total testosterone, sex hormones bound to globulin, and free testosterone was significantly correlated with the severity of OSAHS. This fact corresponds with our study. Singer F36 found that, deprivation of sleep was significantly associated with gonal steroid suppression with a p value less than 0.01 in young individuals who were otherwise normal , but level of luteinizing hormone was not significantly affected. Luboshitzky R et al.37 studied in 10 OSA patient and 5 control and observed that the mean levels and area under the curve of LH and testosterone were higher in controls than in cases (LH in control 43.4 +/- 9.5 vs LH in cases 24.9 +/- 10.2 IU/liter. h with a p valur less than 0.005 and testosterone in controls 113.3 +/- 26.8 vs testosterone in cases 67.2 +/- 11.5 nmol/liter.h with a pvalue less than 0.003. Low serum levels of testosterone have also been identified in diabetic, hyperlipemic, insulin-resistant and / or metabolic syndrome patients so we have excluded all of these from our study. Hence we can say there is a great role of sleep fragmentation and nocturnal hypoxemia in gonadal suppression. It is possible, that in patients of OSAHS pituitary gonadal functions are affected due to fragmented sleep and repeated episodes of hypoxia which affects levels of hormones as observed from our study and most of the previous studies. We have studied impact of OSA on female gonadal steroids in 23 obese female OSA cases and compare with 20 non OSA obese controls of similar age group. We have divided cases and controls into two group on the basis of menstrual history (premenopause or postmenopause) and observed low value of estrogen and progesterone in OSA as compared to control in both the groups and also with the increase in severity of OSA serum estrogen and progesterone decreases (p-value < 0.01) in this study it was found that the correlation between apnea hypoapnea index and levels of estrogen /progesterone was negative correlation ((r = -0.4).. We also found that 60% female in our study were postmenopausal which is congruent to the study of Block, A.J et al.,38 they found that 12 out of 20 postmenopausal females in the study had 102 episodes of SDB and 118 episodes of desaturation of oxygen levels while among premenopausal patients in the study only two out of 18 females had 6 episodes of apnea with a pvalue < 0.01.
Edward O. Bixler et al.9 also observed similar result. They also studied polysomnography in postmenopausal female taking HRT and found that the prevalence in these women were lower than the women who were not taking hormone replacement therapy. Nikolaus C.et al.39 studied in 53 women. 32 women were OSA patient and rest 21 women without OSA taken as control.
Of these 32 women, 20 were menopausal and 12 were pre-menopasual out of which 6 were in the pre-luteal phase/proliferative phase and 6 were in the luteal phase /secretory phase of the menstrual cycle. Of the 21 women without OSAHS, 6 were menopausal, 9 were in the preluteal phase/proliferative phase and 6 were in the luteal phase/secretory phase of the cycle. The progesterone levels in females with OSAHS were less than those without OSHAS both in preluteal / proliferative phase and luteal/secretory phase. The levels of estradiol were lower in menopausal females and females in preluteal/proliferative phase with OSAHS. Female hormones are thought to protect women from OSAS as observed from this and previous studies and vice versa may also be true as this and few previous studies confirm the hypothesis that women with OSAHS have lower levels of progesterone and estradiol. Further larger studies are required todraw a causal relationship between OSAHS and level of hormones but the findings of this study suggests that patients of OSA should be considered for hormone replacement therapy.
Conclusion
This study has shown that increasing OSA severity is associated with lower levels of T4 and higher level of TSH, lower levels of Testosterone, Estrogen, LH and FSH and hence OSA may be associated with functional hypogonadotropic hypogonadism. The recognition and understanding of the complex interactions between hormones and OSAS may allow a multidisciplinary approach to obese patients with these disturbance.to correct the endocrine abnormalities and improve quality of life of patients effective assessment and management is necessary. Further interventional studies are required to determine whether treating these endocrine abnormalities have any effect on the severity of OSA.
Limitations of the Study
Obesity hypoventilation syndrome associated with obese patients in OSA could not be evaluated as ABG was not done to comment on day time hypoxia and hypercarbia.
The effect of OHS on hormonal parameters and quality of sexual life scores could not be evaluated.
Number of subjects included in the study was less so large studies are required for a statistically significant result to be generalized on population.