Introduction
The most prevalent kind of cancer arises from the squamous epithelium of the upper aerodigestive tract. Head and neck cancer affects about 10.3% of all cases in India as in the year 2020. Lip and oral cavity cancers are second most common among the top ten malignancies in India.1 One of the most frequent malignant tumours in the world is oral squamous cell carcinoma. In South East Asia, notably in India, it predominates significantly, accounting for 19% of all male cancer cases and 7% of female cancer cases.2
Variations in OSCC incidence, mortality, location, grade, histological type, and tumour stage at diagnosis have all been linked to differences in gender, race, and age.3 Cancers of the lip mucosa, tongue, gingiva, mouth's floor, palate, and others are considered to be oral cancers.4, 5
Oral squamous cell carcinomas (OSCC), are heterogeneous tumours that arise predominantly as a result of ongoing mutations brought on by genetic instability and environmental effects. The origin of OSCCs might be cancer stem cells, clonal evolution, or a combination of the two.6 The oral squamous cell carcinoma has the grading systems like TNM Staging, Broder’s system and Anneroth’s classification. 7
The cell adhesion molecule known as CD44 is a transmembrane protein that has a role in both cell-to-cell and cell-to-matrix contacts. In addition to this, it is a marker for cancer stem cells and possesses two different phenotypes: 1) The epithelial-mesenchymal transition phenotype, found in cancer stem cells that express the CD44 isoform and are resistant to proteolysis. 2) The cancer stem cells having an epithelial phenotype and express CD44v isoforms that are susceptible to trypsin. This helps in distinguishing between epithelial phenotype cancer stem cells and epithelial-mesenchymal transition phenotype, as epithelial phenotype cancer stem cells have a lower expression of CD44 than epithelial-mesenchymal transition phenotype cells. 8
During the G1, S, G2, and M phases of the cell cycle, the big, non-histone protein known as Ki-67 has been utilised as a marker of proliferative activity. One of the indications for tumour invasion potential and the invasive activity of tumours connected to the degree of malignant neoplastic cells is the degree to which tumour cells express the protein Ki-67 through their proliferative activity. Expression of Ki-67 antigen is elevated in both dysplasia and SCC as compared to normal mucosa. 9
It gives distinct reaction patterns that only involve proliferating cells, immunostaining with antibodies to Ki-67 antigen is well established as a method that is both quick and efficient for evaluating growth fractions of various tumour types.10 Tumours expressing higher Ki-67 carry poor prognosis. The present study was done to evaluate the immunohistochemical expression of CD44 and Ki-67 in histopathologically diagnosed cases of oral squamous cell carcinoma.
Materials and Methods
This Hospital based observational study was conducted at the Department of Pathology. Samples of oral lesions diagnosed as squamous cell carcinoma in the department of Pathology were taken. The study was conducted for duration of 18 month i.e., April 2021 to October 2022. Sixty cases were included in the study.
Study population
Oral lesions, ‘histopathologically diagnosed’ as squamous cell carcinoma, falling within specified duration of time in our hospital, were thoroughly evaluated for tumour grading and IHC expression of Ki-67 and CD 44. Clinical data records, blocks, slides etc were retrieved from archives of histopathology lab, Department of Pathology.
Inclusion criteria
Specimens of oral lesions undergone incisional biopsies and/or radical complete surgical resection diagnosed as squamous cell carcinoma.
Exclusion criteria
Tissues without adequate size and representative material and tissue biopsies of patients who had undergone radiotherapy and /or chemotherapy.
Study method
Histopathological examination
Processing of well labelled specimens were done in our department after fixation of the specimen in 10% neutral buffered formalin for 24-48 hrs. Specimens were grossed, processed, paraffin embedded, appropriate sections were taken and Hematoxylin and eosin stained, then examined under microscope.
Immunohistochemitry
CD44 and Ki-67 were applied on histologically diagnosed oral squamous cell carcinoma. Malignant cells were graded for CD44 membranous staining (Table 1) 9 and malignant cells were graded for Ki-67 nuclear staining (Table 2). 10
Normal tonsil was taken as a positive control for CD44 and lymph node was taken as positive control for Ki-67.
Results
In the present study we studied 60 cases of Squamous Cell Carcinoma. Majority of the subjects belonged to 40-49 years (36.7%) followed by 30-39 years (20.0%), 50-59 years (15.0%) and above 70 years (13.3%). The mean age was 50.90±14.08 years. There were 50 (83.3%) males and 10 (16.7%) females.
Out of 60 cases, Majority of cases i.e. 30 cases (50%) were showing ulcero-proliferative type of growth, followed by 18 cases (30%) showing proliferative growth and rest 12 cases (20%) were having ulcerative type growth.
Maximum number of cases i.e., 32 cases (53.3%) were that of grade 2, followed by 20 cases (33.3%) of grade 1, and 08 cases (13.3%) of grade 3. (Table 3)
On correlation of Ki 67 score with Histological grade, the mean Ki67 percentage was significantly more among poorly differentiated (81.88%±5.94%) compared to moderately differentiated (33.13%±15.44%) which was significantly more than well differentiated (23.50%±9.75%). On contrary, the correlation of CD 44 score with histological grade, the mean CD44 percentage was significantly more among well differentiated (75.50%±11.46%) compared to moderately differentiated (63.28%±25.39%) which was significantly more than poorly differentiated (45.63%±16.57%). Correlation of Ki-67 and CD44 score with histological grades in OSCC was done. (Table 4, Figure 1). Correlation of Ki-67 and CD44 score with histological grades in OSCC revealed p value <0.05 i.e. statistically significant. On comparison, there was statistically significant difference among histological grades.
Figure 1
Photomicrograph showingl A: Well differentiated; B: Moderately differentiated and C: Poorly differentiated oral squamous cell carcinoma (H&E 100x); D: Well differentiated oral squamous cell carcinoma showing immunopositivity for Ki-67 score-1; E: Moderately differentiated oral squamous cell carcinoma showing immunopositivity for Ki-67 score-2; F: Poorly differentiated oral squamous cell carcinoma showing immunopositivity for Ki-67 score- 3; G: Well differentiated oral squamous cell carcinoma showing immunopositivity for CD44 score-4; H: Moderately differentiated oral squamous cell carcinoma showing immunopositivity for CD44 score-2; I: Poorly differentiated oral squamous cell carcinoma showing immunopositivity for CD44 score-2 (IHC 400x) respectively.
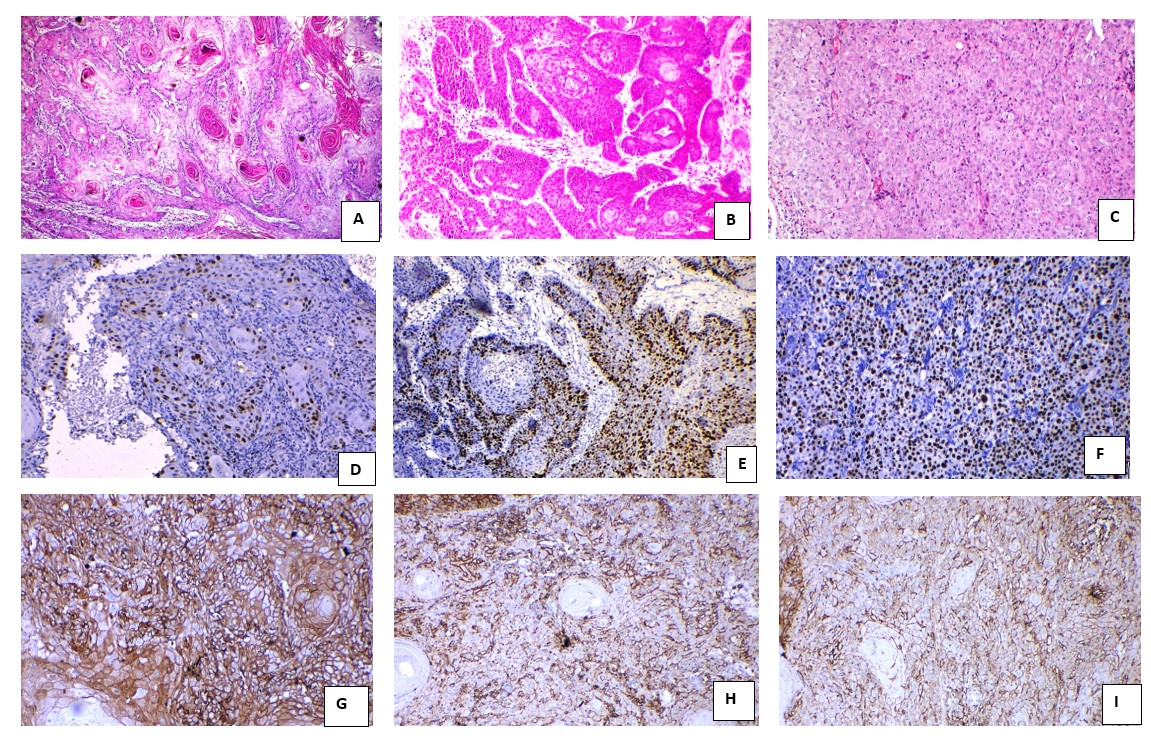
Table 1
Malignant cells were graded for CD44 membranous staining as follows 9
|
Score |
Percentage |
|
0 |
<10% positive cells |
|
1 |
Between 10 and 25% positive cells |
|
2 |
Between 25 and 50% positive cells |
|
3 |
Between 50 and 75% positive cells |
|
4 |
>75% positive cells |
Table 2
Malignant cells were gradedfor Ki-67 nuclear staining (Ki-67 LI LabellingIndex)10 was graded as
|
Score |
Percentage |
|
0 |
LI=0% |
|
1 |
LI=1%-25% |
|
2 |
LI=26%-50% |
|
3 |
LI=51%-75% |
|
4 |
LI=>75% |
Table 3
Distribution of histological grades in OSSC cases
|
Histological Grade |
No. of Cases |
Percentage |
|
Well Differentiated |
20 |
33.3% |
|
Moderately Differentiated |
32 |
53.3% |
|
Poorly Differentiated |
8 |
13.3% |
|
Total |
60 |
100.0% |
Table 4
Correlation of Ki-67 and CD44 score with histological grades in OSCC
Discussion
Squamous cell carcinoma accounts for 95% of the malignant tumors of the oral cavity. More than 60% of the patients present with an advanced stage of the disease. According to Leite and Koifman's research, patients with carcinoma of the tongue died more frequently than those with carcinoma of the lip. 11 In addition, the superior gingivo-facial sulcus is associated with a worse prognosis because of the difficulty in assessing and treating local extension and the abundant lymphatic drainage in that area. 12
Most of the tumors in this study were histological grade II i.e., 53.3% followed by 33.3% in grade I and 13.3% in grade III. The study bears close resemblance to studies done by Wangsa D et al,13 A R Gadbail et al.14 all of which reported maximum cases in grade II followed by grade I and grade III i.e., moderately differentiated OSCC, followed by well differentiated OSCC, followed by poorly differentiated OSCC. The findings were in contrast with the study done by Takkem A et al.15 in which maximum number of cases were seen in grade 3 i.e., poorly differentiated OSCC.
In our study of 60 cases of OSCC, Ki-67 Score 4+ was significantly more among poorly differentiated. The mean Ki-67 percentage was significantly more among Poorly differentiated (81.88%±5.94%) compared to moderately differentiated (33.13%±15.44%) which was significantly more than well differentiated (23.50%±9.75%).
Thirty cases of OSCC were examined by Moghadam S et al16 for Ki-67 expression. For Well differentiated OSCC, the mean Ki-67 was 23.3, for Moderately differentiated OSCC, the mean was 41.2, and for Poorly differentiated OSCC, the mean was 64.1. Takeem A et al15 found that for well differentiated OSCC, the mean Ki-67 protein was 12.7, for moderately differentiated OSCC mean was 30.6, and for poorly differentiated OSCC mean was 53.5, it was determined that the expression of Ki-67 rose steadily with OSCC grade.
Mittal et al17 stated that the average number of positive nuclei per millimetre of length was significantly higher in Oral potentially malignant lesions (OPMLs) with a high proliferation rate than in OPMLs with a low proliferation rate. Within the Malignant lesions, PDSCC had the highest Ki67 LI (66.00 8.4), followed by MDSCC (62.66±8.02) and WDSCC (55.10±6.1). Ki67 LI was shown to be statistically significant throughout all stages of malignancy, which is in line with the findings of Buch A et al 18 and Tumuluri et al.19
Abdelbary et al 20 found that the mean Ki-67 protein was 21.3, for moderately differentiated OSCC mean was 31.3, and for poorly differentiated OSCC mean was 43.5. Verma R et al 21 reported that the mean Ki-67 was 67.33 for Well differentiated OSCC, 75.5 for Moderately differentiated OSCC and 81.45 for Poorly differentiated OSCC.
Tumuluri V et al19 indicated that the histological grading of OSCC and the Ki-67 proliferative indices obtained were linked with each other. Whereas, Stoll et al22 revealed that the Ki-67 index was unable to predict survival in 107 patients with OSCC. These contradictory findings can be related to the tumour type or the immunohistochemical analysis technique (quantitative, qualitative or both). Additionally, population habits may be linked to sample heterogeneity, which could affect how the lesion behaves.
In comparison to well-differentiated OSCC, moderately differentiated OSCC had a greater overall Ki-67 staining.15 Ki-67 staining was diffuse in the majority of the central region of tumour islands, but nuclear Ki-67 positivity was seen at the periphery of poorly differentiated OSCC, indicating that these cells were less differentiated and in a more proliferative phase. 15
Oral squamous cell carcinomas have been linked to heightened expression of the Ki- 67 protein in tumour tissues23 The levels of the protein Ki-67 rise in Oral Epithelial Dysplasia and OSCC as tissue differentiation falls.15 Ki-67 protein is a potential therapeutic target in cancer due to its expression in all proliferating cells and the prognostic value of the Ki-67 marker in many cancers. Strategies that inactivate Ki-67 protein are a promising anti-proliferative approach, with potential application in cancer treatment. 24
Kaza et al25 found that the mean value of CD44 immuno-positive cells was found in well-differentiated squamous cell carcinomas compared to moderately and poorly differentiated squamous cell carcinomas. Immunostaining scores of 3 and 4, indicating the presence of 50-75% and above 75% positive cells, were consistently among well-differentiated squamous cell carcinoma.
Hema KN et al26 revealed that CD44 expression by tumour cells in OSCC was statistically correlated with the tumour grade i.e., Higher mean was observed in well-differentiated squamous cell carcinoma (10.8), followed by Moderately differentiated squamous cell carcinoma (5.9) and poorly differentiated squamous cell carcinoma showed least CD44 immunoexpression (3.7).
Research by Stoll et al 22 and Kanke et al27 all support these results. The degree of cellular proliferation and differentiation was linked to the limited expression of CD44 in different stages of OSCC. The immunostaining expression of CD44 decreases as tumour grade rises, which may indicate a lack of cell-cell adhesion and contribute to the smooth disassembly of cells. Since poorly differentiated squamous cell carcinomas are characterized by highly pleomorphic cells that have little similarity to the parent tissue, low levels of CD44 immunostaining are often seen in these tumours. 25
Moraes et al 28 found that 30 out of 52 cases had positive membranous staining of CD44. The median rate of cell division, as measured by Ki-67, was 37.1% in malignancies. In poorly differentiated tumours in particular, Ki-67 was shown to be expressed all over the parenchyma of the tumour. So, Ki-67 appears to be crucial in the cellular growth of many cancers. Ki-67 has a relationship with both cellular differentiation and proliferation. Cell-cell adhesion, lymphocyte activation, and cell substrate interaction are only a few of the regular uses for the cell membrane molecule CD44. Hyaluronan is bound by CD44. As a result, CD44 may have minimal expression or not function at all, giving the tumour an advantage. 29
Expression of KI-67 and CD 44 has also been studied on cervical squamous cell carcinoma. About 73.1% and 26.9% of cases were strongly positive and positive, respectively, for Ki‑67 expression. 65.4%, 30.8%, and 3.8% of cases were strongly positive, positive, and weakly positive, respectively, for CD44 expression. Ki‑67, and CD44 expression between the three groups were statistically significant. 30
Cˇema et al, revealed that CD44 intra–cytoplasmatic and membranous expression can be recommended as an early indicator of signs of malignant transformation in non-homogeneous leukoplakia and possible loss of stemness. 31
One of the reasons for OSCC therapy failure is the presence of a small pluripotent cell subpopulation identified as “cancer stem cells” (CSCs), which are considered to have a tumour-initiating and self-renewal ability. The transmembrane glycoprotein CD44 has been recognized as a characteristic CSC surface marker that may be used independently or in combination with other markers for the identification of CSCs in various cancers. 32
Conclusion
In order to diagnose OSCC, choose a course of treatment, and determine the prognosis, immunohistochemistry has become a routine procedure. Statistically significant differences among histological grades signifies that immunohistochemistry expression of Ki-67 and CD44 can be used for prognosis and treatment of patients. Thus, the IHC marker CD44 and Ki-67 have been proven to be particularly helpful in predicting the invasive behavior of OSCC. One of the major limitations of this study is small sample size. Thus, further more studies on a larger sample size are needed for generalization of the results of this study.






