Introduction
The US Food and Drug Administration (FDA) in September 2013 approved nab-paclitaxel; the cytotoxic agent as a first-line treatment of patient with metastatic pancreatic adenocarcinoma in combination with gemcitabine.1 In addition, nab-paclitaxel is also approved for its use in locally advanced or metastatic non– small-cell lung cancer as first line treatment in combination with carboplatin and in patients with breast cancer after failure with combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy.2
Paclitaxel, the first taxane was isolated from the bark of pacific yew tree (Taxus brevifolia) as part of a massive plant-screening programme coordinated by the National Cancer Institute and the Department of Agriculture in the United States. 3
Nab-paclitaxel is a solvent-free formulation of paclitaxel that is made by high-pressure homogenization paclitaxel in the presence of serum albumin, resulting in a colloidal suspension containing nanoparticles with an average diameter of 130 nm. This eliminates the possibility of capillary obstruction following intravenous infusion. Nab-paclitaxel is a lyophilized powder that must be reconstituted with 0.9% sodium chloride solution before intravenous use. 4
Paclitaxel inhibits microtubule depolymerization and stabilizes the polymer structure of microtubules thus promoting the synthesis of microtubules in the cells, this decreases free tubulin levels and produces disorganised microtubule clusters. That are incapable for cell replication preventing mitosis. 5, 6 The toxic effects of paclitaxel are most evident in tissues with fast cell turnover, such as alopecia areata-affected tissues, lymphatic, gastrointestinal (GI), and reproductive tissues. 7 Taxane-based chemotherapy regimens have been related to an extensive spectrum of colitis. 8 Diarrhoea (16% to 90%), mucous membrane inflammation (5% to 45%), nausea and vomiting of any severity (9% to 88%), are all paclitaxel-related gastrointestinal symptoms. 9
According to reports, there are 1.2 to 20.3 cases of ulcerative colitis overall per 100,000 people each year, with a prevalence of 7.6 to 245 cases per 100,000 people each year. 10 A study conducted by Ellie Chen et.al revealed that individuals who got docetaxel experienced an incidence of colitis over the 2-year trial period of 1.9%, while individuals who received paclitaxel saw an incidence of 2.5%. Nab-paclitaxel therapy has been related to more extreme colitis than paclitaxel therapy, which results in a milder form of colitis. The average duration between starting taxane and developing colitis symptoms is about 4 weeks. 11
The combination of gemcitabine and nab-paclitaxel is used to treat advanced pancreatic cancer. This treatment may occasionally cause colon (colitis) and pancreas (pancreatitis) irritation. 12 Ulcerative colitis is a relapsing and remitting disease that can affect any part of the colon, beginning with mucosal inflammation in the rectum and progressing proximally. 13, 14 Continuous colonic inflammation that clearly distinguishes between inflamed and non-inflamed intestine is a pathognomonic sign of UC and is characterised by erythema, loss of normal vascular pattern, granularity, erosions, friability, bleeding, and ulcerations. 15
Case History
A 63-year-old patient with a history of hypertension (irregular on medications) and Type-2 Diabetes mellitus arrived at a primary care hospital with complaints of abdominal pain and vomiting. His blood sugar analysis showed HbA1c of 12% and random blood sugar of 171 mg/dL. Liver profiles revealed elevated amylase activity of 220 U/L. An irregularly bordered heterogenous echogenic mass in the pancreatic head area on abdominal sonography raised a suspicion of a neoplastic aetiology. Further examination of the CT scan revealed the presence of pancreatic adenocarcinoma and heterogeneously enhancing lesions in both liver lobes, both of which were most likely metastatic. The same was confirmed by cytology results of a right lobe liver mass and pancreas that revealed a poorly differentiated pancreatic adenocarcinoma that had spread to the liver. The patient was advised to consult an oncologist and was referred to Onco-speciality hospital for further treatment.
On consultation with the oncologist, the patient was diagnosed with stage-IV adenocarcinoma of pancreas with liver metastasis. He was advised for chemotherapy regimen with Gemcitabine and nab-Paclitaxel on day-1 and day-8 for 6 cycles. The treatment was initiated on 5th June 2023, once after the consent was obtained. On completion of Day-1 and Day-8 i.e., Cycle-1 with Gemcitabine and nab-Paclitaxel, three days later the patient started experiencing extreme stomach pain along with vomiting and loose stools that were eventually associated with melena. The patient had consulted a local hospital, as his symptoms started exaggerating, he immediately reported to onco-specialty hospital. On admission the patient was conscious and oriented to place, time and person. On examination his vitals were normal with Abdominal distension and Laboratory investigations of CBC showed decreased platelet count with 0.91 Lakhs. Empirically the patient was treated with intravenous Metronidazole 500mg TID, Ofloxacin 500mg BD, Paracetamol 1g (SOS), Pantoprazole 40mg OD and Ondansetron 4mg (SOS) along with IV-fluids i.e., 1-pint Normal Saline and 1-pint Ringer Lactate. Tab. Clonazepam 0.5 mg+ Escitalopram Oxalate 5 mg OD and Fentanyl patch 25 mcg/hr + 25 mcg/hr (3 mg) were given to avoid anxiety and pain respectively. The patient was constantly followed on the pain scale, and Routine CBC, PT/INR, LFT, and Serum Electrolytes were monitored closely every 4th hourly. The patient was recommended to follow a liquid diet.
On the following day the patient had a sudden hike in his LFT profile (SGPT 95 IU/L), he was advised on Tab. Ursodeoxycholic acid 300mg BD and Syrup. Silymarin 10ml TID. A sigmoidoscopy of the descending colon, sigmoid colon, and rectum revealed erosions, mucosal oedema, and multiple ulcers that were seen indicating severe colitis (as shown in the Figure 1).
Figure 1
Sigmoidoscopy of descending colon and recto-sigmoid region showing multiple ulcers indicating severe colitis.
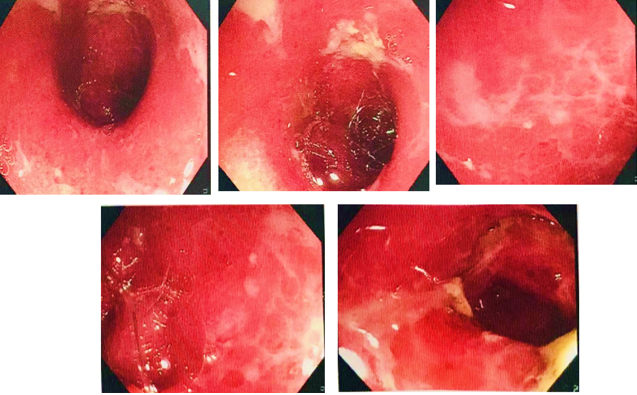
In the view of the gastroenterologist's opinion this is a suspicious case of infection v/s Drug induced colitis. The biopsy findings indicated that the severe active colitis with ulceration was potentially drug-induced. The patient was given Inj. Piperacillin tazobactam 4.5g TID along with Tab. Rifaximin 550mg BD, Tab. Mesalamine 1.2g BD and Tab. Synprotik (pro-prebiotic) TID. The patient's condition gradually improved and was discharged along with these medications Tab. Faronem ER 300mg BD, Tab. Pantoprazole 40mg BD, Tab. Hyoscine butyl bromide 10mg TID, Tab. Tramadol 100mg TID, Capsule Racecadotril 100mg TID, Tab. Tolvaptan 15mg OD HS, Tab. Clonazepam 0.5 mg+ Escitalopram Oxalate 5 mg, Tab. Ondansetron 4mg TID, Tab. Synprotik (pro-prebiotic) TID, Tab. Mesalamine 1.2g BD, Tab. Rifaximin 550mg BD, Syrup. Silymarin 10ml TID for three days.
Discussion
Nab-Paclitaxel is a novel drug used as a first-line treatment for pancreatic adenocarcinoma. It is a solvent-free, albumin-bound 130-nm particle form of paclitaxel that was developed to minimize toxicities associated with the Cremophor vehicle used in solvent-based paclitaxel. Prakash Vishnu et.al compared nab-paclitaxel to traditional paclitaxel, the study showed better safety profiles and higher response rates.16
However, Sara A Hurvitz et.al17 showed The most common gastrointestinal effects due to taxane therapy includes nausea (34-42%), vomiting (22-23%), stomatitis (26-53%), and diarrhoea to severe colitis in rare cases and The most frequently reported nonhematologic side effects (AEs) linked with nab-paclitaxel plus gemcitabine treatment were fatigue (54%), alopecia (50%), and nausea (49%).
A study conducted by Ellie Chen et al11 stated that in comparison to patients who received other taxane, those who received nab-paclitaxel experienced GI toxicity within few days of receiving the drug, needed more hospital stays related to colitis, and required intravenous fluids more frequently. Similarly, this patient developed symptoms of colitis after completing Day-8 Cycle-1 i.e., with a span of 3 days, which had exaggerated lately and required hospitalization. Initially the patient was treated symptomatically later on examination, the Sigmoidoscopy and biopsy reports were suggestive of severe active colitis possibly Drug-induced. The patient was then managed specifically and was discharged on recovery. The patient chemotherapy for the next cycle (i.e., cycle-2) was planned by omitting nab-Paclitaxel and continue with gemcitabine alone.
Conclusion
Patients on nab-Paclitaxel based chemotherapy, presenting with symptoms suggestive of colitis should be monitored to rule out the potential risk of colitis and avoid further exaggeration of the patient’s condition. As taxane-based chemotherapy, especially nab paclitaxel induced colitis is rare and can be potentially fatal. Hence patients require immediate treatment intervention along with aggressive supportive care. The further cycles of chemotherapy should be scheduled as per the patient’s condition, either by dose reduction or by omitting the drug i.e., nab- paclitaxel in this case. Cancer 2004. © 2004 American Cancer Society.






