Panacea Journal of Medical Sciences
Panacea Journal of Medical Sciences (PJMS) open access, peer-reviewed triannually journal publishing since 2011 and is published under auspices of the “NKP Salve Institute of Medical Sciences and Research Centre”. With the aim of faster and better dissemination of knowledge, we will be publishing the article ‘Ahead of Print’ immediately on acceptance. In addition, the journal would allow free access (Open Access) to its contents, which is likely to attract more readers and citations to articles published in PJMS.Manuscripts must be prepared in accordance with “Uniform requiremen...

Bone tumours of the skull: Spectrum of 12 cases with review of literature at tertiary centre in Jaipur
Abstract
Introduction: Skull tumours are usually a mass present next to or inside the skull bones and are benign or malignant and may present with symptoms or exist asymptomatically when diagnosed incidentally. The diagnostic and management options for patients with skull lesions start with complete history and general examination with proper analysis of anatomical location. Appropriate neuro-imaging involving MRI, CT or vascular modalities are used which further refine the differentials of various types of tumours.
Materials and Methods: This is a descriptive observational and cross sectional study done in 15 patients with skull tumours in department of neurosurgery, SMS Medical college and hospital, Jaipur in the academic year: 2022-2023. The clinical history, examination and diagnostic neuro-imaging along with histopathological correlation was studied for reporting of series of 12 cases of skull tumours.
Results: We identified 12 cases of bony tumours of the skull. It was observed that benign tumours (41.7%) were more commonly present than malignant (25%) and intermediate grade (33.3%) ones. The frontal bone was the most common location (66.7%) followed by occipital bone (16.7%). The most common benign tumour was found to be osteoma (25%). Fibrous dysplasia showed intermediate malignant potential and seen in highest proportion in our study (33.3%). Among Malignant tumours, Ewing’s sarcoma was seen in one patient (8.33%) and predominant metastasis in adults with higher age group and in higher proportion (16.7%).
Conclusion: Documentation of systematic series of skull bone tumours is an essential tool in analyzing the differential diagnosis of tumours based on their characteristics of age, sex and location. This case series provides a collective concord for better management of patients. Identification of skull tumours is aided by distinguished use of immuno-histochemistry along with molecular studies and neuro-imaging techniques.
Introduction
Primary bony tumors of the skull are very rare entities (1%) of all bone tumors. They include a large range of lesions (benign or malignant). Skull tumors are rare lesions making up < 2% of all musculoskeletal tumors.[1] They usually present as an enlarged skull mass, symptomatic or asymptomatic.[2] World health organization (WHO) has divided skull tumors into 3 classes: benign, malignant and undefined neoplastic nature tumors (UNNT). Malignant skull tumors are most common ones. They are radiologically categorized as benign tumors with well-defined borders & narrow transition zone with sclerotic margins while malignant tumors show poorly defined margins & wide transition zone, pugnacious periosteal reaction but a soft tissue element which leads to massive bony destruction and intra or extra cranial extension.[3]
Osteomas are the most frequent benign bone moulding tumors which are found in the frontal bone and seen as round sclerotic lesions formed from the outer table of skull without any involvement of diploe on CT scan. The 2nd most frequent tumor among the benign category comprising approximately 10% of benign skull tumors and usually occurs in adults. In CT scans, hemangioma appears as a sunburst pattern. A Brown tumor, known as osteitis fibrosa cystica, very rare clinical tumor, is one of the signs of hyperparathyroidism. It demonstrates a cellular process which is reparative and not neoplastic. Radiologically, lesions are osteolytic having undefined margins. [4] Brown tumors are similar to giant cell tumors (as osteoclastomas) histologically and therefore, they can be misinterpreted in absence of assessment for elevated blood calcium or parathyroid hormone levels.[5]
Fibrous dysplasia, an undefined neoplastic nature tumor (UNNT) mostly seen in younger age, are formed when normal bone is replaced by immature woven bone and seen on CT scan as intradiploic, expansile lesions with ground glass form.[6]
The malignant skull tumors are more common than benign tumors. The malignant tumors of skull includes osteogenic sarcoma, chondrosarcoma, metastases, multiple myelomas and chordoma. Metastases are the most frequent malignant tumors in adults (>50 years) and are secondary to breast, lung, prostate, kidney and thyroid carcinomas and to neuroblastoma or sarcomas in children. They are characterized by multiple osteolytic lesions having a soft tissue element which extends into adjoining tissues. Metastasis from thyroid/renal carcinoma should be considered if lesions are single, expanded & osteolytic. Peripheral primitive neuro-ectodermal tumor, also known as Ewing sarcoma shows varying degree of neuroectodermal differentiation. [6], [7]
These tumors are not collective in literature but only in form of case reports. In our retrospective analysis of the scope of skull tumors, we have documented the range of tumors diagnosed in S.M.S. Hospital Jaipur during one year period (2022-2023) and analyzed their clinico-pathological features.
Material and Methods
Study setting
Department of Neurosurgery, S.M.S. Medical College and Hospital, Jaipur, Rajasthan.
Inclusion criteria
Skull tumor patients presenting in the Department of Neurosurgery during academic year 2022-2023.
Study design
Cross sectional study.
Study type
Descriptive observational study.
Sample size
Patients with skull tumor.
The hematoxylin and eosin-stained slides were recovered from the Department of Pathology and diagnoses were reviewed. The clinical parameters such as age, sex of patient and location of tumor were distinguished.
Results
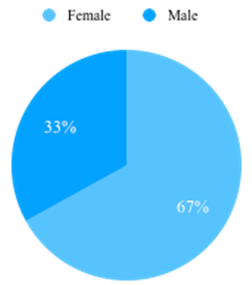
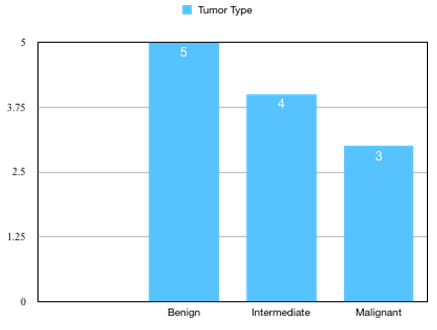
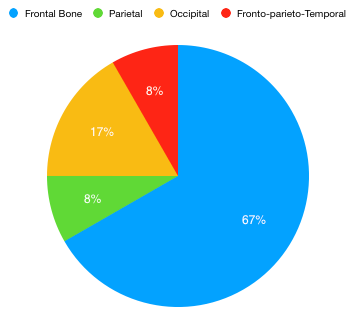
We retrieved twelve cases of bony tumors of skull, which were estimated to be 1.1% of CNS tumors identified during one year period (2022-2023). The mean age range of patients was 12-60 years. Female dominance was observed ([Figure 1]). Patients were mostly adults (9/12; 75%) and pediatric patients were less (3/12; 25%). Benign tumors (5/12;41.7%) were more commonly seen than malignant (3/12; 25%) and intermediate grade (4/12; 33.3%) ([Figure 2]). The frontal bone of the skull was the commonest location (8/12; 66.7%) followed by occipital bone (2/12;16.7%). The Parietal bone was bone was involved in 1 case. In 1 case simultaneously involved parietal, frontal and temporal bone ([Figure 3]).
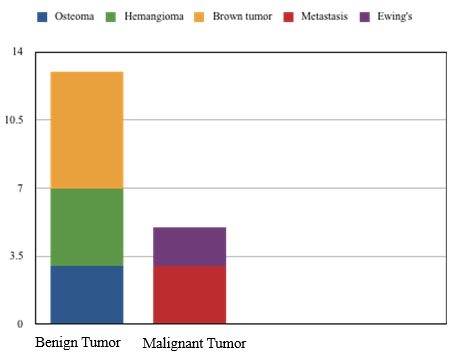
The commonest benign tumor was observed to be osteoma (3/12; 25%). The other benign tumors observed were frontal bone hemangioma and brown tumor ([Figure 4]). Fibrous dysplasia was the one with intermediate malignant activity and was seen in majority (4/12;33.3%). Among malignant, Ewing’s sarcoma/peripheral primitive neuroectodermal tumor was observed in one patient (8.33%) & metastasis present predominantly in adults with higher age group and in higher proportion (16.7%).
Discussion
Bony tumors of the skull are rare and there is paucity of systemically analyzed studies on skull tumors. The bony tumors which were previously at inaccessible locations like base of skull, are now being removed easily with advanced neuro-surgical modalities. The primary tumors of the skull bones are categorized on the basis of origin cell into bone forming tumors, cartilaginous tumors, fibro-osseous tumors and tumors originating from remnants of notochord.
Osteoma
Osteomas are usually benign and slow growing bony tumors, observed in young adult males. Osteomas are non-cancerous slowly growing fibrous tumors having litlle osseous spicules implanted in proliferating connective tissues. The most frequent location is the fronto-ethmoidal region [4] with peak incidence around age of 10-30 years. These are uaually formed in the outer table of skull. The CT scan of osteoma depicts homogenous, well-defined, sclerotic lesions ([Figure 5]). Osteomas are 100% treatable by surgical methods. Gundewar et al found that 25% of craniofacial osteomas were in the orbit and only 17% in the frontal bone. In our study, we diagnosed 3 osteomas in the frontal bone. Multiple osteomas are usually found in patients of Gardner syndrome, intestinal polyps, desmoids & epidermoid cysts. [5], [8]
Brown tumor
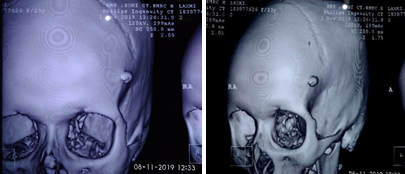
Brown tumor is a benign osseous lesion linked with hyperparathyroidism. It is commonly seen in medullary shaft of long bones & is multifocal or solitary. Khalatbari et al. reported 3 cases of brown tumors of the anterior base of skull as early presentation of true normo-calcemic primary hyper parathyroidism. In the CT scan, they show as well-defined, lytic, uni or multilocular lesions which activate small reactive bone formation. [9]
In this study, we had a 52-year-old male with a swelling on forehead and complaint of frequent headaches since one year. Patient was investigated thoroughly and MRI with NCCT head was done, which depicted expansile lobulated lesion of frontal bone with erosion of outer and inner table of skull and expansion of bone leading to mass effect on bi frontal lobes ([Figure 6]). USG neck showed characteristics of right sided parathyroid mass. Histopathology was suggestive of a giant cell lesion. As the patient was a case of hyperparathyroidism, Brown tumor diagnosis was suspected.
Fibrous dysplasia
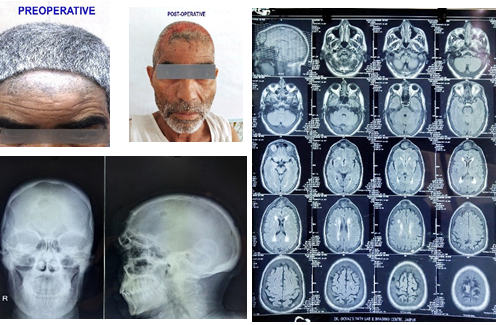
In our study, we found three cases of Fibrous dysplasia. A male aged 20 years, presented with painless swelling over left frontal bone for 3 years. Another patient, a 16-year-old male presented with abnormal swelling over head in frontal region for 4 years and other patient was a 28 year old female who presented with painless swelling over the head involving frontal, parietal and temporal region since last 5 years. In all the patients, thickening of involved bone with expansion was seen with no remnant of any adjacent soft tissue ([Figure 7]). Fibrous dysplasia is mainly identified by immature weak woven bone replacing the normal bone. [8] It is generally seen in childhood and younger age groups (10-30 years). It is usually asymptomatic and painless but because of mass enhancement sometimes, it may present with symptoms and is found in frontal or temporal bone. In a study done by Leeds, [10] 46 patients having Fibrous dysplasia were studied and most common symptom was asymmetrical skull & facial bones. Although the cause is not evident, the molecular studies suggest a mutation in the Gsα subunit and activation of c-fos & other proto-oncogenes. Complete excision of tumor was done followed by Cranioplasty. [10]
Hemangioma
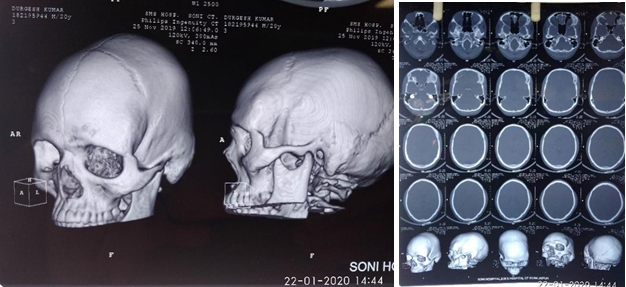
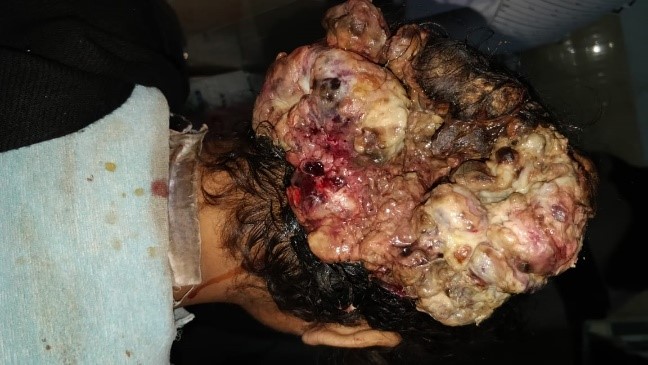
Primary intraosseous cavernous hemangiomas (PICHs) are rare bone tumors. Skull hemangiomas are found in the vault, generally, the frontal bone and predominantly seen in patients in their fourth and fifth decades. Different clinical presentations can be seen in accordance with the involved site Invasion of orbit leads to proptosis and impaired vision. Temporal bone involvement presents as paralysis of facial nerve, oral commissure twitching, pulsatile tinnitus and loss of hearing. Patients may also develop epidural hematoma or subarachnoid hemorrhage. The most common clinical feature was a painful or painless solid swelling in the skull ([Figure 8]). Patients can complain of frequent headaches or dizziness.[11] In our series, one case was seen involving the frontal bone who presented with symptoms of painless swelling overhead since last 5 years. The management for skull PICH is total resection with proper normal bone margins to reduce the bleeding risk. Relapse is rare when adequate safety margins are secured. Other treatment option is curettage then later re canalization and irradiation, to reduce the volume of tumor. It has shown symptomatic improvement, but with radiation-induced carcinoma risk. [11]
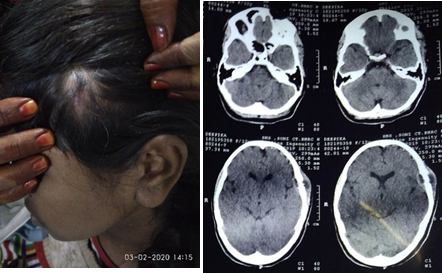
Ewing sarcoma/Peripheral primitive neuroectodermal tumor (ES)
ES is a malignant tumor showing variable degree of neuroectodermal differentiation. It is specified by recurrent balanced translocations of the EWSR1 gene on chromosome 22. It presents in patients of younger age group (<20 years) and found in the diaphysis of long bones. The skull and vertebrae can be involved too. It may arise primarily from the dura, to be misidentified as meningioma on radio images. Histologically, ES is made up of malignant small round cell sheets with scanty cytoplasm containing glycogen, which leads to cytoplasmic clearing of tumor cells. Homer-Wright rosettes can be seen which indicates neuroectodermal differentiation. Distinguishing feature can be diffuse strong membranous positivity for CD99 (MIC2 gene product). On CT imaging, an onion-peel setting with layers of bone mottling and erosion along with new bone formation is observed ([Figure 9]). Surgical removal of the tumor is advised followed by radio and chemotherapy. Prognosis is usually poor to moderate, depending on condition and followed treatment. [12]
|
S.No. |
Sex |
Age |
Histopathology |
Location of tumor |
|
1 |
F |
23 |
Osteoma (Benign) |
Frontal region |
|
2 |
M |
52 |
Brown tumor (Benign) |
Frontal bone |
|
3 |
M |
20 |
Fibrous dysplasia (UNNT) |
Left frontal region |
|
4 |
F |
60 |
Metastases from lung carcinoma (Malignant) |
Frontal bone |
|
5 |
F |
38 |
Ewing’s sarcoma/PNET (Malignant) |
Occipital region |
|
6 |
F |
50 |
Metastases from thyroid follicular carcinoma (Malignant) |
Right occipital region |
|
7 |
M |
16 |
Fibrous dysplasia (UNNT) |
Frontal bone |
|
8 |
F |
18 |
Osteoma (Benign) |
Frontal region |
|
9 |
F |
28 |
Fibrous dysplasia |
Frontal parietal temporal region |
|
10 |
F |
20 |
Fibrous dysplasia |
Right parietal region |
|
11 |
F |
12 |
Frontal bone hemangioma |
Right frontal region |
|
12 |
M |
16 |
Osteoma (Benign) |
Left frontal region |
Metastases
Metastases to the calvarium is commonly seen in cancers. Most commonly brain metastases is observed in breast cancer followed by lung, prostate and lymphoma. Brain metastases are divided into 2 anatomical classes which present distinctive clinical features. One group is calvaria metastasis, generally asymptomatic or may lead to dural invasion, dural sinus occlusion or cosmetic issues. The second group is skull-base metastasis, which presents with cranial-nerve involvement leading to devastating symptoms. A high index of suspicion based on new-onset cranial nerve deficit or craniofacial pain in a cancer patient is important for early diagnosis and prompt management. Magnetic resonance imaging is the primary diagnostic tool. Skull metastasis is a focal lesion with low intensity signal on T1-weighted images. Enhanced T1-weighted images with fat-suppression show tumor, dural infiltration and cranial nerve involvements. Irradiation is the effective and primary treatment for skull metastases. Chemotherapy or hormonal therapy can be used depending on sensitivity of tumors. Bone resorption inhibitory drugs prescribed in systemic therapy are found to be suitable for prevention of symptomatic skull metastasis.
Surgery is done in few patients who require immediate decompression, cosmetic recovery, or have proven histological diagnosis. [13] According to literature, metastases from thyroid is not so frequent. [12] Most common metastases from the thyroid cancer is follicular sub type. [14]
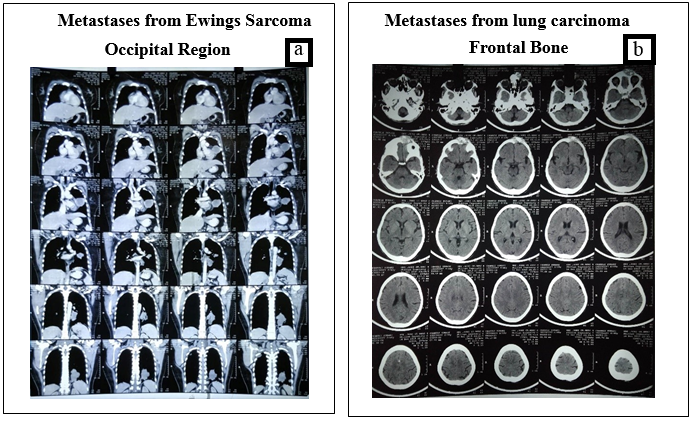
A 50-year-old female patient observed a swelling on her right occipital region since 1 year. Patient was evaluated and diagnosed as a case of right occipital space occupying lesion with extracranial extension. NCCT head revealed soft tissue density with calcific foci causing destruction of occipital bone on the right side along with intracranial extension. Patient underwent right occipital craniotomy with wide local excision. Histopathology showed metastases from follicular thyroid cancer. The literature points out that these tumors are more common in females. The most favourable site for these tumors is midline of the frontal and parietooccipital region. Y Nagamine (1985) et al. [15] in their study found that the incidence of skull metastasis from thyroid cancer was higher among women. The most frequent histopathological presentation was follicular adeno carcinoma. Such lesions were morphologically well differentiated, with little pleomorphism and atypism but detailed examination showed infiltration into the vascular lumen or capsule. The mean survival time in these patients was 4.5 years from the time of diagnosis.
A 60-year-old female developed a painless swelling on forehead since 2 months. CT head depicted destructive soft tissue density lesion along with calcification and erosion of frontal bone, glabella leading to metastases (Image 6&7). On work up for primary, HRCT thorax revealed a malignant mass with spiculated margins in left upper lobe of lung along with calcified foci and multiple enlarged lymph nodes in pre or para tracheal area. USG guided FNAC of frontal area mass depicted poorly differentiated malignant neoplastic cells. Histopathological reports suggested metastases from lung cancer. Lung cancer cells can spread via bloodstream or lymphatics to the brain. Komatsu et al. [16] investigated 70 patients with brain metastases from histologically confirmed lung cancer. Turner et al. [17] encountered a case of lung cancer metastases showing solitary skull lesions. D’Antonio et al. [18] reported a review demonstrating the newer advancements in therapeutic management of bone and brain metastases in lung carcinomas. Pain is the first symptom of lung cancer with bone metastases in 80% of patients. [19] Patients with osseous metastases complain of pain at some point with wide variation in pattern and severity. [20] Many factors are implicated in the pain of osseous metastases but a significant portion of the pain seems to be related to osteoclastic bone resorption. Surgery, irradiation, stereo-tactic resection and chemotherapy have proven beneficial to treat such patients.
Conclusion
Documentation of systematic series of skull bone tumors is an essential tool in analyzing the differential diagnosis of tumors based on their characteristics of age, sex & location. This case series provides a collective concord for better management of patients.
Source of Funding
None.
Conflict of Interest
None.
References
- Liu H, Zhang X, Zhang M, Zhang J, Ning W, Yue A. Skull bone tumor: a review of clinicopathological and neuroimaging characteristics of 426 cases at a single center. Cancer Commun. 2019;39(1). [Google Scholar] [Crossref]
- Geschickter C. Primary Tumors of the Cranial Bones. Am J Cancer. 1936;26(1):155-80. [Google Scholar]
- MG, Colglazier E, Paulk K, Vogel H, Guzman R, Edwards M. Primary pediatric skull tumors. Pediatr Neurosurg. 2011;47(3):198-203. [Google Scholar]
- Smith M, Calcaterra TC. Frontal sinus osteoma. Ann Otol Rhinol Laryngol. 1989;98(11):896-900. [Google Scholar]
- Parisien M, Silverberg S, Shane E, Dempster D, Bilezikian J. Bone disease in primary hyperparathyroidism. Endocrinol Metab Clin North Am. 1990;19(1):19-34. [Google Scholar]
- Celenk P, Zengin Z, Muglali M, Celenk C. Computed tomography of cranio-facial fibrous dysplasia. Eur J Radiol. 2009;69(3):85-7. [Google Scholar]
- Kakkar A, Nambirajan A, Suri V, Sarkar C, Kale S, Singh M. Primary Bone Tumors of the Skull: Spectrum of 125 Cases, with Review of Literature. J Neurol Surg B Skull Base. 2016;77(4):319-25. [Google Scholar]
- Gundewar S, Kothari D, Mokal N, Ghalme A. Osteomas of the craniofacial region: A case series and review of literature. Indian J Plast Surg. 2013;46(3):479-85. [Google Scholar]
- Khalatbari M, Hamidi M, Moharamzad Y, Setayesh A, Amirjamshidi A. Brown tumors of the anterior skull base as the initial manifestation of true normocalcemic primary hyperparathyroidism: report of three cases and review of the literature. Turk Neurosurg. 2013;23(2):260-6. [Google Scholar]
- Leeds N, Seaman W. Fibrous dysplasia of the skull and its differential diagnosis. A clinical and roentgenographic study of 46 cases. Radiology. 1962;78:570-82. [Google Scholar] [Crossref]
- Salunke P, Sinha R, Khandelwal NK. Primary intraosseus cavernous hemangioma of the skull base. Br J Neurosurg. 2010;24(1):84-5. [Google Scholar]
- Parekh S, Donthineni-Rao R, Ricchetti E, Lackman R. Fibrous dysplasia. J Am Acad Orthop Surg. 2004;12(5):305-13. [Google Scholar]
- Gibiezaite S, Ozdemir S, Shuja S, Mccook B, Plazarte M, Sheikh-Ali M. Unexpected Bone Metastases from Thyroid Cancer. Case Rep Endocrinol. 2015. [Google Scholar] [Crossref]
- Shen J, Wang S, Zhao X, Shao X, Jiang X, Dai Y. Skull metastasis from follicular thyroid carcinoma: report of three cases and review of literature. Int J Clin Exp Pathol. 2015;8(11):15285-93. [Google Scholar]
- Nagamine Y, Suzuki J, Katakura R, Yoshimoto T, Matoba N, Takaya K. Skull metastasis of thyroid carcinoma. Study of 12 cases. J Neurosurg. 1985;63(4):526-31. [Google Scholar]
- Komatsu T, Kunieda E, Oizumi Y, Tamai Y, Akiba T. Clinical characteristics of brain metastases from lung cancer according to histological type: Pretreatment evaluation and survival following whole-brain radiotherapy. Mol Clin Oncol. 2013;1(4):692-8. [Google Scholar]
- Turner R, Lucke-Wold B, Hwang R, Underwood B. . J Surg Case Rep. 2016;2016(6). [Google Scholar] [Crossref]
- D'Antonio C, Passaro A, Gori B, Signore E, Migliorino M, Ricciardi S. Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol. 2014;6(3):101-14. [Google Scholar]
- Kosteva J, Langer C. The changing landscape of the medical management of skeletal metastases in nonsmall cell lung cancer. Curr Opin Oncol. 2008;20(2):155-61. [Google Scholar]
- Delaney A, Fleetwood-Walker S, Colvin L, Fallon M. Translational medicine: cancer pain mechanisms and management. Br J Anaesth. 2008;101(1):87-94. [Google Scholar]
Article Metrics
- Visibility 11 Views
- Downloads 3 Views
- DOI 10.18231/pjms.v.15.i.1.18-24
-
CrossMark
- Citation
- Received Date June 03, 2024
- Accepted Date November 11, 2024
- Publication Date March 12, 2025