Panacea Journal of Medical Sciences
Panacea Journal of Medical Sciences (PJMS) open access, peer-reviewed triannually journal publishing since 2011 and is published under auspices of the “NKP Salve Institute of Medical Sciences and Research Centre”. With the aim of faster and better dissemination of knowledge, we will be publishing the article ‘Ahead of Print’ immediately on acceptance. In addition, the journal would allow free access (Open Access) to its contents, which is likely to attract more readers and citations to articles published in PJMS.Manuscripts must be prepared in accordance with “Uniform requiremen...

Clinicopathological profile of benign soft tissue tumors- A study of 256 cases
Abstract
Introduction: Soft tissue tumors are heterogenous group of tumors with diverse histology and similar clinical and radiological features. Benign soft tissue tumors outnumber sarcomas by a wide margin.
Aim and Objectives: To perform category-wise analysis of benign soft tissue tumors and correlate histopathological distribution with clinical parameters.
Materials and Methods: This was a retrospective type of study carried out over a period of four and half years. Histopathological examination of all the excisional biopsies of benign soft tissue tumors was performed. The tumors were classified as per WHO classification of soft tissue tumors 2013. The data was represented in the form of frequency and percentage. Analysis of histopathological findings was performed followed by correlation of histopathological findings with clinical parameters.
Results: A total of 256 benign soft tissue tumors were studied. These tumors were most commonly seen in fourth decade of life and were more common in males with male to female ratio of 1.15: 1. Trunk was the most common site followed by upper extremity. Adipocytic tumors (215 cases, 84%) were most common among all the tumors, followed by nerve sheath tumors (27 cases 10.5%) and vascular tumors (12 cases 4.7%). There was one case each of benign fibrous histiocytoma and glomus tumor.
Conclusion: Adipocytic tumors form the bulk of benign soft tissue tumors. But there is a considerable variety among the remaining small proportion of these tumors. Most of these have peculiarities regarding the age, gender and site-wise distribution.
Introduction
Soft tissue tumors are defined as tumors arising from non-epithelial extraskeletal tissue of the body excluding the reticuloendothelial system, glia and supporting tissue of various parenchymal organs.[1] The diagnosis of soft tissue tumors is based on histologic findings and rapidly advancing ancillary tests like immunohistochemistry and molecular methods like reverse transcriptase polymerase chain reaction, fluorescence in situ hybridization, and more recently next generation sequencing. Molecular alterations can also be efficiently detected by immunohistochemical markers which can be protein correlates of molecular genetic alterations protein products of gene fusion or diagnostic markers identified by gene expression profiling. [2]
Histopathological analysis shows that benign soft tissue tumors significantly outnumber the malignant soft tissue tumors. [3] Our study aims at performing category-wise analysis of benign soft tissue tumors and correlation of histopathological findings with clinical parameters.
Materials and Methods
Study center and ethical approval
This is a histopathological study of benign soft tissue tumors carried out at our tertiary care center after approval by ethics committee.
Sample size- 256.
Study duration and design
Four and half years, retrospective.
Inclusion criteria
All the excisional biopsies of benign soft tissue tumors were included.
Exclusion criteria
Incisional biopsies were excluded from our study. All the cases of malignant and intermediate grade soft tissue tumors were excluded from the study.
Methodology
Clinical data including history, physical examination findings and relevant investigations were noted from the histopathology requisition forms and the case records of the patients. The specimens received were fixed in 10% formalin. A detailed gross examination was done including size and shape, borders and circumscription, consistency, cut surface and secondary changes of tumors. Representative tissue bits from different parts of soft tissue tumors were submitted for histopathological processing. Thin tissue sections of three to four micron thickness were cut by microtome and stained with Hematoxylin and Eosin stain. Special stains like Reticulin and Masson trichrome stain were used wherever required. Microscopic examination was performed. Immunohistochemistry was performed in selected cases, wherever essential. The tumors were classified as per WHO classification of soft tissue tumors 2013.[3] The data was represented in the form of frequency and percentage. Analysis of histopathological findings was performed followed by correlation of histopathological findings with clinical parameters.
Results
The present study included a total of 256 cases of benign soft tissue tumors. These tumors were most commonly seen in fourth decade of life followed by fifth decade and were more common in males with male to female ratio of 1.15: 1 ([Table 1])
|
Age |
Male |
Female |
Total |
|
0-10 |
03 |
02 |
05 (1.95%) |
|
11-20 |
04 |
06 |
10 (3.91%) |
|
21-30 |
24 |
20 |
44 (17.19%) |
|
31-40 |
24 |
34 |
58 (22.65%) |
|
41-50 |
25 |
23 |
48 (18.75%) |
|
51-60 |
21 |
18 |
39 (15.23%) |
|
61-70 |
29 |
13 |
42 (16.41%) |
|
71-80 |
07 |
03 |
10 (3.91%) |
|
Total |
137 (53.52%) |
119 (46.48%) |
256 (100%) |
Overall, trunk was found to be the most common site followed by upper extremity ([Table 2]).
|
Site |
Number |
Percentage (%) |
|
Upper Extremity |
57 |
22.3 |
|
Lower Extremity |
33 |
12.9 |
|
Trunk |
111 |
43.4 |
|
Head And Neck |
52 |
20.3 |
|
Retroperitoneum |
01 |
0.4 |
|
Multiple |
02 |
0.8 |
|
Total |
256 |
100 |
Category-wise distribution of the tumors ([Table 3]) shows that adipocytic tumors were most common among all the tumors
|
Category |
Number |
Percentage |
|
Adipocytic |
215 |
84 |
|
Nerve Sheath |
27 |
10.5 |
|
Vascular |
12 |
4.7 |
|
Fibro histiocytic |
01 |
0.4 |
|
Pericytic |
01 |
0.4 |
|
Total |
256 |
100 |
Benign adipocytic tumors
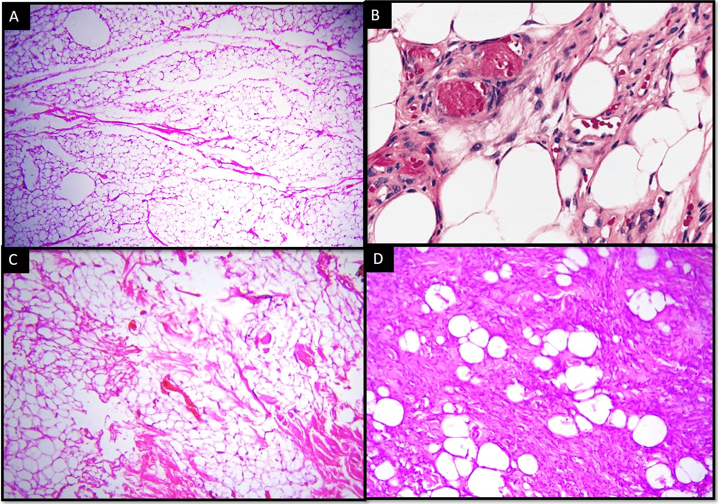
Out of 215 cases of benign adipocytic tumors, conventional lipoma constituted about 196 cases (91.16%) of benign adipocytic tumors. Other variants found were angiolipoma (12 cases, 5.58%), spindle cell lipoma (6 cases, 2.79%) and myolipoma (1 case, 0.47%). [[Figure 1]]
All the cases had presented with palpable mass. Few cases of angiolipoma also had pain associated with swelling. Most common age group for conventional lipoma was fourth and fifth decades. It was more common in males (53.06 %) than in females (46.94%). Trunk was found to be the most common anatomical site (51%). For angiolipoma, third decade was the most common age group with 50% of cases. Angiolipoma was more commonly seen in males. Almost all cases of angiolipoma were found over extremities (83%). The age range for spindle cell lipomas was from third to eighth decades, frequency being twice in males as compared to females. Most common site for spindle cell lipoma was head and neck (50%).
Benign vascular tumors
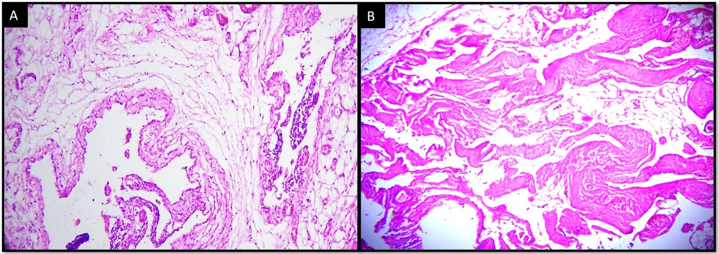
We found 12 cases of benign vascular tumors, including lymphangioma (6 cases, 50%), arteriovenous malformation (4 cases, 33.33%) and intramuscular hemangioma (2 cases, 16,67%). [[Figure 2]]
There was an equal distribution among males and females. The vascular tumors were more common in the first three decades of life (66%). Head and neck was the commonest site for vascular tumors (58.3%). Patients with lymphangioma had presented with cystic lesion while those with arteriovenous malformation presented with pulsatile lesion.
Peripheral nerve sheath tumors
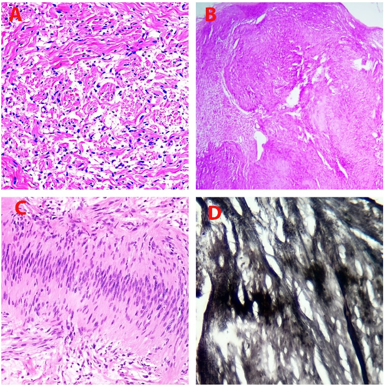
Total 27 peripheral nerve sheath tumors were found including 13(48.15%) Neurofibromas, 13(48.15%) Schwannomas [[Figure 3]] and one (3.70%) Perineurioma.. All the patients had presented with subcutaneous nodule with or without associated pain. Overall, nerve sheath tumors were most common in the age group of 21-30 years (66.7%) upper extremity being the commonest anatomical location (37% cases). Neurofibroma and schwannoma were more common in males than in females. Neurofibroma was most common in upper extremity and trunk (38.5% each). Schwannoma was most commonly found in head and neck region (46.2%), followed by upper extremity (30.8%).
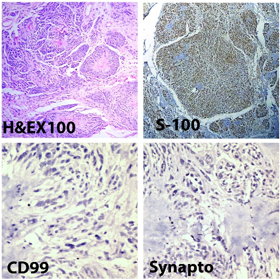
We reported one case of neuroblastoma like schwannoma in a 19 years girl, who had presented with nodular swelling over left ankle. Immunohistochemistry showed strong and diffuse positivity for S 100 protein. Synaptophysin and CD99 immunostaining was negative, which ruled out the diagnosis of neuroblastoma or primitive neuroectodermal tumor ([Figure 4]). The patient was diagnosed as a case of schwannomatosis and had multiple subcutaneous nodules over extremities, abdomen and trunk since 10 years.
There was a case of plexiform schwannoma in our study. The patient was a 28 years male who presented with superficial swelling over forearm.
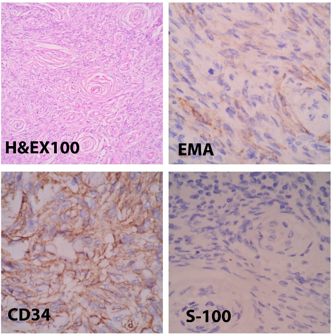
Perineurioma was diagnosed in a 32 years male patient. Histopathology showed tumor cells arranged in perivascular whorls and storiform pattern. On Immunohistochemistry, the tumor cells showed positive staining with CD 34 and EMA and were negative for S-100 which confirmed the diagnosis of Perineurioma. ([Figure 5])
Fibrohistiocytic tumors
Benign fibrous histiocytoma was the only tumor among fibrohistiocytic category which was found in single case in present study. It was seen in neck region. Patient was nine years male child.
Pericytic (perivascular) tumors
In the present study we found single case of benign pericytic tumor i.e. Glomus tumor. The patient was 66 years old male who presented with swelling over right elbow.
Discussion
Out of the 256 cases of benign soft tissue tumors in our study, the maximum frequency of benign soft tissue tumors was found in fourth decade (22.65%) followed by fifth decade (18.75%), male to female ratio being 1.15:1. Male predominance for benign soft tissue tumors has been reported by other studies as well. [4], [5] Trunk was found to be the most common site (43.4%) followed by upper extremity (22.3%) in our study. Ramnani et al [4] who studied 120 cases of benign soft tissue tumors found maximum cases in fourth decade (27.5%) followed by third decade (22.5%). The most common site in their study was trunk (25%), followed by upper extremities (21.7%).
The mean age of patients for benign soft tissue tumors was found to be 36.6 ± 17.7 years in the study by Singh et al. [5] The most common sites in their study were head and neck (55cases) followed by upper extremity (48cases), out of the total 181 cases. [5]
[Table 4] shows comparison ofcategory wise distribution of benign soft tissue tumors in different studies.
Histopathological analysis of the tumors in different studies shows that adipocytic tumors were most common in all the studies followed by vascular tumors and nerve sheath tumors. In our study, nerve sheath tumors were more common than vascular tumors in contrast to other studies.
|
Study |
Soni et al[6] 2014 |
Singh et al [5] 2017 |
Sharma et al[7] 2020 |
Present study |
|
Category |
||||
|
Adipocytic |
68 (48.6%) |
79 (43.6%) |
60 (53.1) |
215 (84%) |
|
Fibroblastic/ Myofibroblastic |
10 (7.1%) |
8 (4.4%) |
12 (10.6%) |
- |
|
Fibrohistiocytic |
11 (7.9%) |
10(5.5%) |
- |
01 (0.4%) |
|
Pericytic (perivascular) |
01 (0.7%) |
3 (1.7%) |
- |
01(0.4%) |
|
Vascular |
24 (17.1%) |
48 (26.5%) |
30 (26.5%) |
12 (4.7%) |
|
Nerve sheath |
23 (16.4%) |
27 (14.9%) |
11 (9.7%) |
27 (10.5%) |
|
Muscular |
02 (1.4%) |
02 (1.1%) |
- |
- |
|
Chondro- osseous |
01(0.7%) |
- |
- |
- |
|
Other |
- |
04 (2.2%) |
- |
- |
|
Total |
140 (100%) |
181(100%) |
113 (100%) |
256(100%) |
Most of the benign adipocytic tumors in our study were conventional lipomas (196 cases, 91.16%) and variants like angiolipoma (12 cases), spindle cell lipoma (6 cases) and myolipoma (1 case). Bera K et al [8] reported conventional lipoma (74 cases), angiolipoma (3 cases), spindle cell lipoma (1 case), myolipoma (1 case) and fibrolipoma (5 cases). Naik et al [9] in their study of soft tissue tumors found that out of 63 lipomas 58 (92.1%) were classical lipomatous tumors, whereas 5(5.9%) were fibrolipomas. They found lipomas most frequently in the fourth decade of life with upper extremity being the commonest site. Lin et al [10] studied 459 cases of lipomas, that included 388 conventional lipomas, 25 angiolipomas, 39 fibrolipomas, 3 myxolipomas and 4 hibernomas. Of the 25 angiolipomas in their study, 23 were noninfiltrating, while two were infiltrating type. All the angiolipomas in our study were noninfiltrating type.
In our study, trunk was found to be the most common site for conventional lipoma (51%). Almost all cases of angiolipoma were found on extremities (83%). Most common site for spindle cell lipoma was head and neck (50%). Naik et al [9] found upper extremity as the most common site for lipoma followed by trunk. Punia et al [11] studied 12 cases of angiolipoma, including eleven males and one female. Pain/tenderness was present in all except one. The locations were upper extremities or trunk. Chen et al [12] studied 40 cases of spindle cell lipomas that included 9 females and 31 males. The mean age was 54 years. The common sites were head, neck, shoulder and back.
The peripheral nerve sheath tumors accounted for 27 cases in our study including 13 Neurofibromas, 13 Schwannomas and one Perineurioma. Overall, nerve sheath tumors were most common in the age group of 21-30 years (66.7%). Neurofibroma and schwannoma were more common in males than in females in our study. Naik et al [9] encountered 15 benign peripheral nerve sheath tumors among the 108 benign soft tissue tumors in their study, out of which, 12 tumors (80%) were neurofibromas and three tumors were schwannomas. Neurofibromas occurred mainly in males (60%) in the seventh decade of life. Schwannomas, occurred in second, third and seventh decades of life, commonly in females (66.6%)
Patil et al [13] studied 27 cases of neurofibroma with a broad age-range (10-74 years) with female preponderance (59.2% in females), of which, 59.2% were conventional neurofibromas, 11.1% were plexiform and 29.6% were diffuse neurofibromas. The common locations were extremities and head and neck region (62.9%) in their study, whereas upper extremity and trunk (38.5% each) were the most common sites for neurofibroma in our study. In their study, five cases with conventional neurofibroma, three cases with plexiform neurofibroma and four cases of diffuse neurofibroma had Neurofibromatosis 1 (NF1). We did not encounter any case of NF1.
In our study, schwannoma was most commonly found in head and neck region (46.2%), followed by upper extremity (30.8%). Naik et al[9] also reported head and neck region as the commonest site for schwannomas. Mujumdar et al [14] who studied 12 cases of Schwannomas reported upper limbs (55.55%) as the commonest site.
We diagnosed a case of plexiform schwannoma in our study. There was no evidence of any syndromic association. Berg et al [15] noted that plexiform schwannoma represented 4.3% of all schwannomas, and 5% of these were associated with neurofibromatosis type 2 and schwannomatosis each. We also reported one neuroblastoma like schwannoma in a case of schwannomatosis. This is a very rare variant of schwannoma, that can be misdiagnosed as neuroblastoma or primitive neuroectodermal tumor.[16]
Perineuriomas need to be differentiated from neurofibromas, schwannomas or hybrid schwannoma- perineurioma. [17] We had performed immunohistochemistry study in our case. The tumor cells showed positive staining for EMA and CD34 and were negative for S-100, thus confirming the diagnosis.
The vascular tumors encountered in our study were lymphangioma (6 cases), arteriovenous malformation (4 cases) and intramuscular haemangioma (2 cases). These were more common in the first three decades of life (66%) and there was an equal distribution among males and females. Head and neck was the commonest site for vascular tumors (58.3%). Naik et al[9] also noted head, neck and face as the most common sites for hemangiomas, 7(33.3%) out of 21 cases. Kalyani et al [18] in their study of vascular lesions found that benign vascular lesions were more common in females (46 cases) as compared to males (31 cases) Pyogenic granulomas were the commonest benign vascular tumors followed by capillary hemangioma and cavernous hemangiomas in their study.
In our study, for lymphangioma, four out of six patients were females, three out of six patients were in first or second decades of life and head and neck was most common site (3 cases), followed by trunk 3 (2 cases). Kang et al [19] studied 51 patients of lymphangioma. They observed that lymphangiomas predominantly occurred in females. In their study, 56.9% of the patients were under 10 years age, mean age being 16.5 years and trunk (43.6%), was the most common site followed by extremities (30.9%) and head and neck. (20.0%).
The rarely found tumors in our study were benign fibrous histiocytoma and glomus tumor which were relatively rare in other studies as well.
Conclusion
To conclude, though lipomas form the bulk of benign soft tissue tumors, there is a considerable variety among the remaining small proportion of these tumors. Most of these have some peculiarities regarding the age, gender and site-wise distribution. Knowledge of the clinicopathological profile can be helpful to the clinician for provisional diagnosis and further management of soft tissue tumors. But histopathology is the ultimate gold standard.
Conflict of Interest
None.
Source of Funding
None.
References
- Goldblum J, Folpe A, Weiss S. . Enzinger & Weiss’s soft tissue tumors. 2020. [Google Scholar]
- Choi J, Ro J. The Recent Advances in Molecular Diagnosis of Soft Tissue Tumors. Int J Mol Sci. 2023;24(6). [Google Scholar] [Crossref]
- Fletcher C, Bridge J, Hogendoorn P, Mertens F. . WHO Classification of Tumors of Soft Tissue and Bone. 2013. [Google Scholar]
- Ramnani B, Kumar A, Chandak S, Ranjan A, Patel M. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):1-4. [Google Scholar]
- Singh H, Grover S, Garg B, Sood N. Histopathological Spectrum of Soft-Tissue Tumors with Immunohistochemistry Correlation and FNCLCC grading: A North Indian Experience. Niger Med J. 2017;58(5):149-55. [Google Scholar]
- Soni P, Verma A, Chandoke R, Nigam J. A prospective study of soft tissue tumors histocytopathology correlation. Patholog Res Int. 2014. [Google Scholar] [Crossref]
- Sharma P, Mahajan M, Gupta R, Bharadwaj S. Histopathological pattern of soft tissue tumors in a tertiary care centre in North India: A retrospective study. Int J Med Biomed Stud. 2020;4(3):25-7. [Google Scholar]
- Bera K, Thaker MV. A study of pattern of distribution of soft tissue tumors in a population of Bhavnagar district. IOSR J Dent Med Sci. 2016;15(6):57-60. [Google Scholar]
- Naik V, Hooger M, Sahu S, Khadayate R. Histomorphological Profile and Clinicopathological Correlation of Soft Tissue Tumours- A Study at a Tertiary Care Teaching Hospital. Int J Health Sci Res. 2018;8(9):35-42. [Google Scholar]
- Lin J, Lin F. Two entities in angiolipoma. A study of 459 cases of lipoma with review of literature on infiltrating angiolipoma. Cancer. 1974;34(3):720-7. [Google Scholar]
- Punia R, Jain P, Amanjit, Mohan H, Singh R. Subcutaneous angiolipomas: a clinicopathological study of 12 cases. Indian J Pathol Microbiol. 2005;48(2):197-8. [Google Scholar]
- Chen S, Huang H, He S, Wang W, Zhao R, Li L. Spindle cell lipoma: clinicopathologic characterization of 40 cases. Int J Clin Exp Pathol. 2019;12(7):2613-21. [Google Scholar]
- Patil S, KC. A histopathological study of neurofibroma and malignant peripheral nerve sheath tumors in a medical institute of Central Karnataka. Int J Clin Diagn Pathol. 2021;4(1):81-5. [Google Scholar]
- Majumder A, Ahuja A, Chauhan D, Paliwal P, Bhardwaj M. A clinicopathological study of peripheral schwannomas. Med Pharm Rep. 2021;94(2):191-6. [Google Scholar]
- Berg J, Scheithauer B, Spinner R, Allen C, Koutlas I. Plexiform schwannoma: a clinicopathologic overview with emphasis on the head and neck region. Hum Pathol. 2008;39(5):633-40. [Google Scholar]
- NS, Valaeys V, Geerts M, André J. Neuroblastoma-like schwannoma: a case report and review of the literature. Am J Dermatopathol. 2003;25(1):32-4. [Google Scholar]
- Belakhoua S, Rodriguez F. Diagnostic Pathology of Tumors of Peripheral Nerve. Neurosurgery. 2021;88(3):443-56. [Google Scholar]
- Kalyani D, Bharatrao N. Histopathological study of vascular lesions. IOSR J Dent Med Sci. 2017;16(11):47-52. [Google Scholar]
- Kang B, Kang H, Kim S, Kim J, Park Y, Oh S. A clinicopathologic study of lymphangioma. Korean J Dermatol. 2006;44(9):1044-50. [Google Scholar]
- Abstract
- Introduction
- Materials and Methods
- Study center and ethical approval
- Study duration and design
- Inclusion criteria
- Exclusion criteria
- Methodology
- Results
- Benign adipocytic tumors
- Benign vascular tumors
- Peripheral nerve sheath tumors
- Fibrohistiocytic tumors
- Pericytic (perivascular) tumors
- Discussion
- Conclusion
- Conflict of Interest
- Source of Funding
- References
Article Metrics
- Visibility 8 Views
- Downloads 3 Views
- DOI 10.18231/pjms.v.15.i.1.66-71
-
CrossMark
- Citation
- Received Date October 28, 2023
- Accepted Date December 08, 2023
- Publication Date March 12, 2025