Panacea Journal of Medical Sciences
Panacea Journal of Medical Sciences (PJMS) open access, peer-reviewed triannually journal publishing since 2011 and is published under auspices of the “NKP Salve Institute of Medical Sciences and Research Centre”. With the aim of faster and better dissemination of knowledge, we will be publishing the article ‘Ahead of Print’ immediately on acceptance. In addition, the journal would allow free access (Open Access) to its contents, which is likely to attract more readers and citations to articles published in PJMS.Manuscripts must be prepared in accordance with “Uniform requiremen...

Adiponectin levels and their association with obesity and type 2 diabetes mellitus
Abstract
Background: Adipose tissue metabolism has become area of research specially in obesity and its correlation with diabetes. It is now widely acknowledged that in addition to its conventional job as a store of energy, adipose tissue is a vital and extremely active endocrine organ that generates a range of hormones and other chemicals that are crucial for controlling insulin sensitivity and other physiological functions. This study looked into the connection between type 2 diabetes mellitus (T2DM), obesity, and adiponectin levels.
Materials and Methods: Researchers recruited 120 participants and divided them into four groups: control, obese non-diabetic, diabetic with normal BMI, and obese diabetic. Serum adiponectin levels, HbA1c, postprandial blood glucose, and fasting blood glucose were measured from blood samples. In order to determine BMI and the waist-to-hip ratio, measurements of height, weight, waist, and hip circumference were taken as well.
Results: The study found that adiponectin levels were significantly lower in all obese and diabetic groups compared to the control group (5.3±0.64 ng/ml, 6.11±1.09 ng/ml, 5.2±0.75 ng/ml and 7.99±2.5 ng/ml respectively). A negative correlation was observed between adiponectin levels and both BMI and HbA1c. These findings suggest a link between low adiponectin, obesity, and T2DM.
Conclusion: This study highlights the potential role of adiponectin in understanding the development of T2DM in obese individuals. Further research on the mechanisms underlying this relationship may lead to novel therapeutic targets for obesity and T2DM.
Introduction
Technology is advancing at a breakneck pace, causing abrupt lifestyle shifts and a noticeable rise in non-communicable diseases like obesity and diabetes, as well as its comorbidities, worldwide. A group of common metabolic illnesses are together referred to as diabetes mellitus.[1] Uncontrolled diabetes often leads to hyperglycaemia, which over time causes significant harm to several bodily systems. The prevalence of overweight and obesity, together with poor lifestyle choices, are the main causes of the high and rising worldwide burden of diabetes, particularly in emerging nations like India. [1]
According to a 2019 World Health Organization (WHO) estimate, noncommunicable diseases (NCDs) accounted for 74% of global deaths, with diabetes accounting for 1.6 million of those deaths.[2] By 2035, it is predicted that diabetes will likely claim the lives of 592 million people worldwide. The proportion of diabetics in India rose from 7.1% in 2009 to 8.9% in 2019 The prevalence is expected to increase to 35.7 million by 2045. After China, India has the second-highest number of diabetes cases globally, with 77 million cases. [2]
Multifactorial in origin, diabetes mellitus is a modifiable disease.[3] Obesity is a major risk factor for the development of diabetes mellitus. For several chronic conditions, including Type II Diabetes Mellitus, Hypertension, and Hypercholesterolemia, it is an effective predictor of morbidity and mortality. Excess calorie consumption and physical inactivity contribute to obesity.[3] Similar to diabetes, type 2 diabetes associated with obesity most likely has many aetiologies. A number of interrelated factors, including body fat distribution, β-cell malfunction, insulin resistance, and physical inactivity, contribute to the development of type II diabetes. [4]
Since the start of the twenty-first century, the incidence of obesity has increased, raising concerns about its potential to endanger public health worldwide. The NHFS survey (2019–2021) revealed that the prevalence of abdominal obesity in the country was 40% in women and 12% in men. [5] Approximately 44% of the adult population, or over 2 billion adults, are overweight or obese, and the majority of them—70%—live in low- and middle-income countries (LMICs).
Researchers' interest in adipose tissue metabolism has been piqued by the concerning increase in obesity prevalence and its correlation with diabetes. It is now widely acknowledged that adipose tissue serves as an important and extremely active endocrine organ that generates a range of hormones and other chemicals that are crucial for controlling insulin sensitivity and other physiological functions, in addition to its traditional function as an energy storage depot (Havel 2002). [6] Recently, about 600 hormones and proteins generated from adipose tissue have been found. These substances are referred to as adipokines. White adipose tissue secretes a variety of proteins, including adipsin, angiotensin, retinol-binding protein, acylation-stimulating protein, tissue factor, resisting, metallothionein, plasminogen activator inhibitor-1, interleukin-6, tumour necrosis factor-α, adiponectin, and many more.[7] In response to inflammation and malfunction of adipose tissue, adipokine production is markedly altered towards a pro-inflammatory and diabetogenic pattern. [8]
A new protein exclusive to adipocytes is called adiponectin.[9] It is essentially an adipokine that directly increases the body's sensitivity to insulin and is expressed particularly and abundantly in adipose tissue.[10], [11], [12], [13] It is a protein hormone that controls several metabolic functions, such as the breakdown of fatty acids and the regulation of glucose.[13] The release of adiponectin is restricted by factors that are known to be increased in insulin resistance, including glucocorticoids, β-adrenergic agonists, TNF-α, and IL-6.[14] The adiponectin gene is expressed more when thiazolidinediones are present.[15] It improves weight loss, insulin sensitivity, and glucose tolerance.[12] In human research, low levels of adiponectin plasma are positively associated with insulin resistance and the risk of type 2 diabetes, independent of body fat amount.[16] Adiponectin also demonstrates anti-inflammatory and anti-atherogenic qualities.[17]
The adiponectin gene is one that appears to be a strong candidate for type 2 diabetes risk. Genetic studies have connected this to the metabolic syndrome and Type 2 Diabetes Mellitus. Therefore, SNPs in the adiponectin gene may be connected to insulin resistance and type 2 diabetes through modifications in the expression level and plasma concentration of adiponectin. [18]
The hormone is a particularly attractive target for potential therapeutic approaches centered on the idea that adiponectin treatment may alleviate obesity-related insulin resistance and atherosclerosis because obesity is the state of adiponectin shortage. Our study sought to assess the levels of adiponectin in obese diabetics and diabetic patients with normal body mass index in order to investigate the role of adiponectin in the pathophysiology of diabetes and obesity.
Materials and Methods
This is prospective, case control study. It has been conducted in Department of Biochemistry, at Tertiary care hospital, Navi Mumbai. The aim of study is to estimate Fasting blood glucose (FBS) and post prandial blood glucose (PPBS), HbA1c and serum adiponectin levels in healthy (non-obese) and Study Groups. Ethical clearance was taken from scientific and ethical committee of the institution. Patients gave their written and informed consent prior to study participants being included.
In all, total120 people in the 25–60 age range who were matched for both gender and age were registered.30 normal healthy individuals were included in control group. Rest 90 individuals included in study group which were further subdivided into 3 sub-groups: (30 individuals in each sub-groups) Study group I, Study group II and Study group III. Group I comprise of obese and non-diabetic individuals. Non-obese diabetic and Obese diabetic individuals were included in Group II and III respectively.
All participants provided demographic information, a clinical history, and anthropometric measurements, such as height (cm) and weight (kg). The height and weight were measured to the nearest 0.1 kg and 0.5 cm, respectively. The BMI was computed by dividing weight (kg) by height (m) squared. Individuals with a BMI greater than 30 were classified as obese. The waist-to-hip ratio (WHR) was calculated by dividing waist circumference by hip circumference.
Control group comprise of healthy individuals whereas Obese individual without Diabetes were included in Study group I. Diabetic patient with normal BMI (BMI ≤ 25) and Obese (BMI ≥ 30) Diabetic subjects were included in Study Group II and III. Individuals beyond study age, Individuals taking Thiazolidienediones, known / suspected pregnancy, Cancer, with history of CVD, Asthma, overweight individuals with BMI between 25 to 30 were excluded.
After 12 hours overnight fast, 6ml of blood was collected from each subject by venipuncture using aseptic phlebotomy technique in a plain vial for serum separation, sodium fluoride vial for plasma and EDTA vial for HbA1c estimation. Plasma was collected again after two hours of post meal for the postprandial glucose (PPBS) estimation. Serum was used for Adiponectin assay. Serum samples were stored at -70oC till analysis.
The Biochemical parameters such as fasting blood sugar (FBS) & (PPBS) were analysed on Biochemistry Autoanalyser (AU480). HbA1c estimation has been done on Bio-Rad D10. Serum Adiponectin was estimated by ELISA method using commercially available Avibion Human Adiponectin (Acrp 30) ELISA kit.
Statistical analysis
Data reported were statistically analysed by R-software which is freely available online.
Result
A total of 120 individuals were categorised into 4 groups. 30 participants in each group. Control group consist of 65% males and 35% females. Out of 90 individuals in study group, 53% were males and 47% were females.
|
Groups |
BMI (kg/m2) Mean ± S.D. |
WHR Mean ± S.D. |
|
Control |
22.5±1.6 |
0.89±.05 |
|
Study Group I |
32.2±2.56** |
0.99±.05** |
|
Study Group II |
23.1±1.33# |
0.91±.04* |
|
Study Group III |
33.1±3.1** |
0.98±.004** |
|
Groups |
FBS (mg/dL) Mean ± S.D. |
PPBS (mg/dL) Mean ± S.D. |
HbA1c (%) Mean ± S.D. |
|
Control |
95.3±6.7 |
127±5.38 |
5.19±2.2 |
|
Study Group I |
95.7±9.5# |
127.6±8.4# |
5.2±0.64# |
|
Study Group II |
123±20.9** |
198±24.2** |
8.07±2.8** |
|
Study Group III |
129±22.5** |
226±23.8** |
8.13±1.4** |
|
Groups |
Adiponectin (ng/ml) Mean ± S.D. |
|
Control |
7.99±2.5 |
|
Study Group I |
5.3±0.64** |
|
Study Group II |
6.11± 1.09** |
|
Study Group III |
5.2±0.75** |
|
|
Study Group I |
Study Group II |
Study Group III |
|
Adiponectin & BMI |
-0.22 |
-0.31 |
-0.34 |
|
Adiponectin & HbA1c |
-0.19 |
-0.20 |
-0.28 |
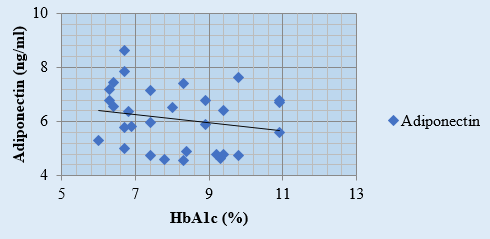
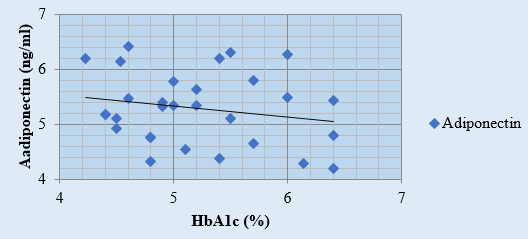
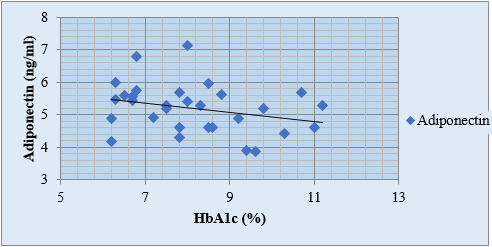
Discussion
Type 2 diabetes is a diverse illness caused by a complex interplay between environmental and genetic factors.[19] Obesity is closely associated with the polygenic syndrome that most people with type 2 diabetes have. Among the lifestyle factors that raise the risk of diabetes mellitus include a poor diet, stress, urbanization, and inactivity.[20] Type 2 diabetes mellitus (T2DM) and obesity are two different global health problems; however, they have been found to be related. People with diabetes are frequently overweight and obese.[21]
The incidence of overweight and obesity is increasing at a faster rate than traditional issues like undernutrition and infectious diseases. Comorbidities associated with obesity include dyslipidaemia, gout, osteoarthritis, respiratory diseases, sleep apnoea, gallbladder disease, non-insulin-dependent diabetic mellitus, coronary heart disease, hypertension, stroke, and certain cancers.[3]
We investigated adiponectin as a potential marker to understand its role in the pathogenesis of obesity and diabetes. The levels of adiponectin have been estimated in Obese individuals without diabetes (Group I), non-obese diabetic individuals (Group II) and obese diabetic (Group III) individuals.
DM type II is commonly seen associated with obesity. As a result, early obesity detection can aid in avoiding associated problems. Anthropometric indices are simple, inexpensive, and non-invasive indicators for monitoring obesity and body fat distribution. Consistent with this, our study found that Study Group I (obese) had significantly higher BMI and WHR (32.2±2.56 kg/m2 & 0.99±.05) compared to control group (22.5±1.6 kg/m2 & 0.89±.05) with p ≤ 0.001. Group III showed statistically significant BMI and WHR compared to control with p ≤ 0.001(Table 1).
BMI (kg/m2) is the most widely used statistic for defining overweight and obesity. It is a straightforward weight-for-height index. It is computed by dividing the weight of an individual in kilograms by the square of his height in meters (kg/m2).[3] According to the WHO, a person is considered overweight if their BMI is 25 or higher, and obese if their BMI is 30 or higher. An additional metric for evaluating the risk of obesity and its repercussions is the waist-hip ratio (WHR). In addition to being a more practical measure, the waist circumference also indicates a person's likelihood of acquiring type 2 diabetes and cardiovascular disease.[22] According to Vague's 1956 description, people with an android-shaped (central) of fat distribution were more likely to have health problems than people with a gynoid-shaped (peripheral) of fat distribution.[4] Insulin resistance is independently correlated with central adiposity.[4] Those without obesity frequently have a high waist-to-hip ratio. Increased body mass index, fat distribution, weight increase rate, and ectopic fat accumulation are all potential causes of type 2 diabetes in obese people. [4], [21]
Diabetic status was assessed by estimating FBS and PPBS, and HbA1c in study and control group. [Table 2] shows results of fasting and post- prandial plasma sugar levels in control and study groups. In comparison to the control group, both diabetic groups (Group II and III) had significantly significant fasting and postprandial plasma sugar levels (p < 0.001). Additionally, group I and the control group did not vary significantly (p > 0.05). ([Table 2]) Comparing groups II and III to the control group, there was a significantly significant relationship for HbA1c (p < 0.001). As anticipated, there is no discernible difference between group I and the control group (p > 0.05).
A reliable indicator of sustained glucose management is HbA1c. As haemoglobin glycation is dependent on plasma glucose and is not enzymatic, the study groups with diabetes reported increased HbA1c values. The current diagnostic cut-off for fasting and 2-hour postprandial plasma glucose levels are correlated with HbA1c values ≥ 6.5%. However, the majority of people with HbA1c levels between 6.0% and 7.0% had normal fasting plasma glucose levels and abnormal 2-hour post prandial plasma glucose levels. HbA1c readings help guide glucose control, show patient compliance, and have a strong correlation with the emergence of diabetic complications. [4]
Blood pressure, inflammation, haemostasis, endothelial function, insulin sensitivity and secretion, fat distribution, appetite and satiety, and energy expenditure are all regulated by adipokines.[23] Serum Adipokines levels are measured to assess obesity and associated health problems and to comprehend the pathophysiology of diabetes in order to create methods for therapy and follow-up. Serum levels of Adiponectin were measured for both the study and control groups.
[Table 3] shows serum adiponectin levels in the control and study groups. Adiponectin levels were found to be lower in all study groups than in the control group (p < 0.001). In comparison to the control group, which had an average of 7.99±2.5 ng/ml, the levels of adiponectin in groups I, II, and III were 5.3±0.64 ng/ml, 6.11± 1.09 ng/ml, and 5.2± 0.75 ng/ml, respectively. Our results in study groups I and III were in accordance with clinical research by Arita et al. 1999, [10] Hotta et al. 2001,[24] and Yang et al. 2001, [25] which likewise shown that obese people had lower levels of adiponectin than lean people. These studies also reported an improvement in adiponectin levels after an intervention of weight loss. elevated adiponectin levels are associated with leanness. [24], [25]
Adiponectin levels were much greater in malnourished anorexics with drastically reduced body fat content than in age- and gender-matched controls, according to a study by Delporte et al. (2002).[26] Reduced circulating levels of adiponectin were shown to be more strongly associated with the degree of adult insulin resistance and hyperinsulinemia than with the degree of obesity and glucose intolerance, according to a study by Hu et al. [27]
As a pro-inflammatory state, obesity typically manifests as low-grade chronic inflammation linked to elevated levels of pro-inflammatory cytokines, which have a positive correlation with body mass index (BMI). The most prevalent adipokine in serum, adiponectin, has anti-inflammatory properties that guard against cardiovascular and metabolic disorders. Unlike other adipokines, it is lessened in obesity, which lowers the risk of metabolic syndrome, atherosclerosis, insulin resistance, hypertension, fatty liver, and coronary artery disease.[21], [28] Negative correlation has been observed between BMI and adiponectin in all study groups. The r value for adiponectin and BMI in control group was -0.19, whereas in the study groups I, II and III was -0.22, -0.31 and -0.34 respectively. ([Table 4])
Our finding of negative correlation between BMI and Adiponectin were consistent with the study done by Maeda et al in 2001,[29] Ferris WF et al.[30] and Prakash J, et al. (2013)[28]
As BMI rises, so does the risk of Type II diabetes. It starts at a relative risk of 1 at a BMI of 22 kg/m2, rises to 2 to 8 times at a BMI of 25 kg/m2, and then exponentially escalates to 40 to 80 times at a BMI >35 kg/m2, according to Chan J M et al.[31] BMI is not a good predictor of type 2 diabetes risk because only 50% of people with a BMI of over 40 kg/m2 will develop diabetes.[29] Significant genetic predisposing factors may put certain people at higher risk even if their BMI is relatively low. It implies that BMI is not the only important predictor of diabetes risk. [3]
Adiponectin and the development of type 2 diabetes have been shown to be significantly correlated by research (Lindsay et al., 2002).[32] Additionally, it has been demonstrated that hypoadiponectinemia has a stronger independent and substantial correlation with metabolic syndrome than any other inflammatory marker (Matsushita et al., 2006). A number of diseases that are commonly associated with insulin resistance, such as hypertension (Adamczak et al., 2003), dyslipidemia (Matsushita et al., 2006), and cardiovascular disease (Matsuzawa, 2010; Pischon et al., 2004), also typically exhibit decreased plasma adiponectin levels. [33], [34]
A negative correlation between HbA1c and adiponectin levels has been observed, with r value for adiponectin and HbA1c in the study groups I, II and III was -0.19, -0.20 and -0.28 respectively. ([Figure 1], [Figure 2], [Figure 3], [Table 4]). Arita et al., 1999;[10] Matsuzawa, 2010 have also reported an inverse relation between adiponectin and HbA1c.[33], [34] According to study by Neuparth M J, et al., 2013, adiponectin levels were lower in diabetic patients but are more strongly related to obesity which corroborates with our results as well.[21] According to Hotta K. et al.'s 2000 study, [24] adiponectin levels in type 2 diabetes are negatively correlated, regardless of BMI. This conclusion is consistent with our comparison of study groups II and III, which include obese diabetics and those with normal BMI.
One of the fundamental characteristics of these illnesses is insulin resistance, which is characterized as a condition in which glucose homeostasis requires higher than normal insulin levels to be maintained. Hyperinsulinemia results from an increase in insulin production to counteract this insulin resistance. It is evident that obesity is associated with both insulin resistance and hyperinsulinemia. The diminished cellular insulin response caused by insulin resistance, which also results in insufficient control of gluconeogenesis in the liver and decreased glucose absorption in skeletal muscle, is "the vicious circle" of type 2 diabetes. [33] Insulin's effects depend on its dosage and duration of action, and it is a key modulator of the adiponectin gene's synthesis.[35]
While insulin does not correlate with adiponectin levels, type 2 diabetes lowers adiponectin levels, and both diabetics and non-diabetics have a negative correlation between adiponectin levels and body mass index (Hotta et al. 2000). In mice models of obesity and diabetes, adiponectin infusion reduces blood glucose levels without altering insulin levels. (Berg et al., 2001). [12] The main mechanism underlying adiponectin's glucose-lowering effect is increased insulin-suppression of hepatic glucose output (Berg et al., 2001; Combs et al., 2001). [12]
Diseases associated with obesity are characterized by impaired adiponectin activity. Obesity is linked to hypoadiponectinemia and decreased levels of the adiponectin receptors AdipoR1 and AdipoR2. Type 2 diabetes and atherosclerosis may be caused by AdipoR1-mediated reduction of adiponectin activities in the liver, muscle, and macrophages. Impaired adiponectin activity via AdipoR1 and AdipoR2 in liver and cancer cells may be the cause of fatty liver and cancer. Compromised adiponectin activity via AdipoR2 in endothelial cells may lead to atherosclerosis. [33]
Adiponectin decreases the release of pro-inflammatory cytokines IL-6, IL-8, and MCP-1 by encouraging muscle glucose uptake and fatty acid oxidation in muscle, liver, and peripheral tissues. [36] The hormone is a very desirable target for potential therapeutic approaches centred on the idea that adiponectin treatment may alleviate obesity-related insulin resistance and atherosclerosis because obesity is the state of adiponectin scarcity.[37]
Conclusion
Our findings unequivocally demonstrate a strong association between adiponectin levels, obesity, and an increased incidence of type II diabetes mellitus. Gaining insight into the mechanism behind this relationship could improve our comprehension of the pathophysiology of type II diabetes and the diverse nature of obesity. Indians may be more vulnerable because of the dysregulation of these traits. Adiponectin levels may be a precursor to adipocyte dysfunction and have been associated with type II diabetes mellitus. Actions done to prevent obesity through individual and societal lifestyle changes may halt the current epidemic of type II diabetes mellitus.
Conflict of Interest
None.
Source of Funding
None.
References
- Braunwald F, Kasper, Hauser, Longo J, s, U, et al. . Harrison’s Principles of Internal Medicine. 2008;2. [Google Scholar]
- Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69(11):2932-8. [Google Scholar]
- . . . . [Google Scholar]
- AB. . Diabetes: Best Practice & Research Compendium. 2011. [Google Scholar]
- Chaudhary M, Sharma P. Abdominal obesity in India: analysis of the National Family Health Survey-5 (2019-2021) data. Lancet Reg Health Southeast Asia. 2023;14. [Google Scholar] [Crossref]
- Havel P. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002;13(1):51-9. [Google Scholar]
- Trayhurn P, Beattie J. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60(3):329-39. [Google Scholar]
- Blüher M. Clinical relevance of adipokines. Diabetes Metab J. 2012;36(5):317-27. [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Mat-Subara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (Adipose most abundant Gene transcript 1). Biochem Biophys Res Commun. 1996;221(2):286-9. [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79-83. [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941-6. [Google Scholar]
- Berg A, Combs T, Du X, Brownlee M, Scherer P. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7(8):947-53. [Google Scholar]
- Fruebis J, Tsao T, Javorschi S, Ebbets-Reed D, Erickson M, Yen F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98(4):2005-10. [Google Scholar]
- Brunn J, Lihn A, Verdich C, Pedersen S, Toubro S, Astrup A. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285(3):527-33. [Google Scholar]
- Combs T, Wagner J, Berger J, Doebber T, Wang W, Zhang B. Induction of adipocyte complement-related protein of 30 kilodaltonsby PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143(3):998-1007. [Google Scholar]
- Tschrtter O, Fristche A, Thamer C, Haap M, Shirkavand F, Rahe S. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52(2):239-43. [Google Scholar]
- Hara K, Boutin P, Mori Y, Tobe K, Dina C, Yasuda K. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51(2):536-40. [Google Scholar]
- Burtis C, Ashwood E, Burns D. . Teitz Textbook of Clinical Chemistry and Molecular Diagnostics. 2012. [Google Scholar]
- Banday M, Sameer A, Nissar S. Pathophysiology of diabetes: An overview. Avicenna J Med. 2020;10(4):174-88. [Google Scholar]
- Al-Goblan A, MA, Khan M. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587-91. [Google Scholar] [Crossref]
- Neuparth M, Proneca J, Silva AS, Coimbra S. Adipokines, oxidized low-density lipoprotein, and C-reactive protein levels in lean, overweight, and obese portuguese patients with type 2 diabetes. ISRN Obesity. 2013. [Google Scholar] [Crossref]
- Chan J, Rimm E, Colditz G, Stampfer M, Willett W. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17(9):961-9. [Google Scholar]
- Blüher M. Adipokines - removing road blocks to obesity and diabetes therapy. Mol Metab. 2014;3(3):230-40. [Google Scholar]
- Hotta K, Funahashi T, Bodkin N, Ortmeyer H, Arita Y, Hansen B. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50(5):1126-33. [Google Scholar]
- Yang W, Lee W, Funahashi T, Tanaka S, Matsuzawa Y, Chao C. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86(8):3815-9. [Google Scholar]
- Delporte M, Funahash T, Takahashi M, Matsuzawa Y, Brichard S. Pre- and post-translational negative effect of beta-adrenoceptor agonists on adiponectin secretion: in vitro and in vivo studies. Biochem J. 2002;367(Pt 3):677-85. [Google Scholar]
- Hu E, Liang P, Speigleman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271(18):10697-703. [Google Scholar]
- Prakash J, Mittal B, Awasthi S, Agarwal C, Srivastava N. Hypoadiponectinemia in obesity: association with insulin resistance. Ind J Clin Biochem. 2013;28(2):158-63. [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagare-Tani H. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8(7):731-7. [Google Scholar]
- Ferris W, Naran N, Crowther N, Rheeder P, Merwe LVD, Chetty N. The relationship between insulin sensitivity and serum adiponectin levels in three population groups. Horm Metab Res. 2005;37(11):695-701. [Google Scholar]
- Chan J, Rimm E, Colditz G, Stampfer M, Willett W. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17(9):961-9. [Google Scholar]
- Lindsay R, Funahashi T, Hanson R, Matsuzawa Y, Tanaka S, Tataranni P. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360(9326):57-8. [Google Scholar]
- Yamuchi T, Kadowaki T. Adiponectin Receptor as a Key Player in Healthy Longevity and Obesity-Related Diseases. Cell Metab. 2013;17(2):185-96. [Google Scholar]
- Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(2):131-41. [Google Scholar]
- TK, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784-92. [Google Scholar]
- Giby V, Ajith T. Role of adipokines and peroxisome proliferator-activated receptors in nonalcoholic fatty liver disease. World J Hepatol. 2014;6(8):570-9. [Google Scholar]
- Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -indepen-dent pathways. J Biol Chem. 2006;281(13):8748-55. [Google Scholar]
Article Metrics
- Visibility 8 Views
- Downloads 2 Views
- DOI 10.18231/pjms.v.15.i.1.81-87
-
CrossMark
- Citation
- Received Date June 23, 2024
- Accepted Date November 21, 2024
- Publication Date March 12, 2025