Panacea Journal of Medical Sciences
Panacea Journal of Medical Sciences (PJMS) open access, peer-reviewed triannually journal publishing since 2011 and is published under auspices of the “NKP Salve Institute of Medical Sciences and Research Centre”. With the aim of faster and better dissemination of knowledge, we will be publishing the article ‘Ahead of Print’ immediately on acceptance. In addition, the journal would allow free access (Open Access) to its contents, which is likely to attract more readers and citations to articles published in PJMS.Manuscripts must be prepared in accordance with “Uniform requiremen...

Role of intravenous labetalol in the treatment of severe pre eclampsia and eclampsia
Abstract
Background: Hypertensive disorder of pregnancy complicates about 5-10% of all pregnancies. Severe preeclampsia and eclampsia are the severe forms of hypertension leading to multisystem dysfunction. Early identification of the disease and prevention of complications helps in improved maternal and fetal outcome. Labetalol is a potential drug used in the treatment of acute hypertension. It is used in treating preeclampsia to prevent eclampsia. Hence we would like to study the safety and efficacy of intermittent intravenous Labetalol in the control of severe hypertension in pregnancy and its effect on fetomaternal outcome.
Materials and Methods: This cross sectional study was done in the Department of Obstetrics and Gynaecology, Gandhi Medical College from December 2010 to September 2012. A total of 100 patients diagnosed as severe preeclampsia and eclampsia are included in the study. Primigravida and multigravida with severe preeclampsia, eclampsia, gestational age >20 weeks and postpartum eclampsia were included in the study. All the patients who met inclusion criteria were given intravenous labetalol of 20mg and repeated the dose if the blood pressure still found to be high after 15 minutes. Monitoring continued till target blood pressure achieved till termination of pregnancy.
Results: In my study 79% of women had severe preeclampsia and 21% had eclampsia. The study showed 59% of women were primigravida and majority were of young age. 80% of women showed very high blood pressure recordings between 160/110 - 180/110 mmHg. Only 3.8% of women with blood pressure > 160/110 mmHg were given MgSO4 after giving intravenous labetalol due to presence of imminent signs. 74% of women showed Grade I fundal changes and 5% had retinal detachment. Among the severe pre eclamptic women, the mean difference of reduction in the systolic and diastolic blood pressure after ten minutes of giving intravenous labetalol was highly significant (p < 0.001). Following intravenous Labetalol only 1.2% developed eclampsia.
Conclusion: Labetalol has improved efficacy to MgSO4 with lack for intensive monitoring of blood magnesium levels and suitability for use by primary health care personnel. The study proves it is devoid of maternal and foetal side effects with good perinatal outcome.
Introduction
The tragic problem of maternal death has attracted greater attention over last 15 years. National Health Policy 2002 states that maternal mortality should be reduced to 100 per 1,00,000 live births and perinatal mortality to 30 per thousand live births by 2010. Half of the maternal deaths from complications of pregnancy related hypertension are preventable.[1] The main aim of the obstetrician should be to achieve this objective for safe mother and childhood.
Hypertensive disorders of pregnancy complicate about 5-10 % of all pregnancies.[2], [3] and preeclampsia accounts for 3.9% of obstetric cases.[4] Preeclampsia is not preventable but eclampsia is preventable. Severe dysfunction of renal, pulmonary, hepatic, CNS systems occurs as a consequence of preeclampsia which warrant multidisciplinary management and treatment. Early recognition and treatment is associated with improved maternal and fetal outcome.
Labetalol has been used for many years to safely treat hypertension in preeclamptic women. Intravenous Labetalol is recommended as one of the first line agents in the management of acute severe hypertensive disorder. Labetalol hydrochloride combines both selective competitive alpha adrenergic blocking and non-selective competitive, beta adrenergic blocking activity in a single substance.[4] In man , the ratios of alpha to beta blockade have been estimated to be approximately 1:3 and 1:7 following oral and intravenous IV administration respectively.[5] Since Labetalol has low toxicity potential, does not require specialized training to administer or monitor, it may be an ideal agent for controlling blood pressure (BP) and decreasing the incidence of eclampsia in women with preeclampsia. To date, there have not been many clinical trials regarding intravenous Labetalol in pregnancy. Hence we would like to study the efficacy of intermittent intravenous Labetalol in the control of severe hypertension in pregnancy.[6]
Aims and Objectives
To study the safety and efficacy of intravenous Labetalol in severe preeclampsia and eclampsia.
To assess the time taken and the number of doses required to achieve target blood pressure of ≤ 150/100 mmHg.
To study the effect of intravenous Labetalol on maternal and fetal outcome.
Materials and Methods
This study was done in Department of Obstetrics and Gynaecology Gandhi Medical College from December 2010 to September 2012. A total of 100 patients diagnosed as severe preeclampsia and eclampsia are included in the study. This is a Cross Sectional study done before and after giving Intravenous Labetalol.
Primigravida and multigravida with severe preeclampsia- blood pressure >160/110 mmHg, eclamptic patients, gestational age >20 weeks and post-partum eclampsia were included in the study. Patients with history of asthma, congestive heart failure, heart block, underlying liver disease,epilepsy complicating pregnancy and peripheral vascular disease, gestational age <20 weeks were excluded from the study.
All the patients who met the inclusion criterion were investigated for the laboratory parameters in the antepartum period like Complete Blood Picture, Renal Function test , Liver Function Test, Coagulation Profile and Fundoscopy. They were given 20mg intravenous labetalol if there was a sustained blood pressure of ≥ 160 mmHg/110 mmHg on repeat measurements 15 minutes apart. If repeat blood pressure recorded after 10 minutes was still >160/110 mm Hg, a dose of 40 mg slow I.V over 2 minutes was given. Blood pressure was rechecked and if it still persisted >160/110mmHg another double dose of 80mg slow I.V over 2 minutes was given. Woman was placed in the supine position for at least 2hrs following intravenous Labetalol to avoid postural hypotension. During the study period maternal blood pressures are recorded at every fifteen minutes interval till first 30 minutes after achieving target blood pressure less than or equal to 150/100, then every thirty minutes for next 2 hours then every hourly. The end point in the study was until the termination of pregnancy.
Results
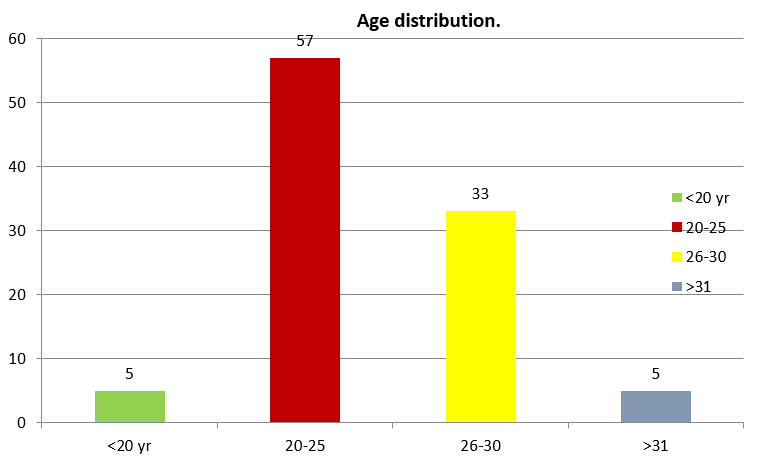
In my study 79% of women had severe preeclampsia and 21% had eclampsia. 57% of women with severe preeclampsia were between 20-25 years age group and 33% were between 26 – 30 years. ([Figure 1])
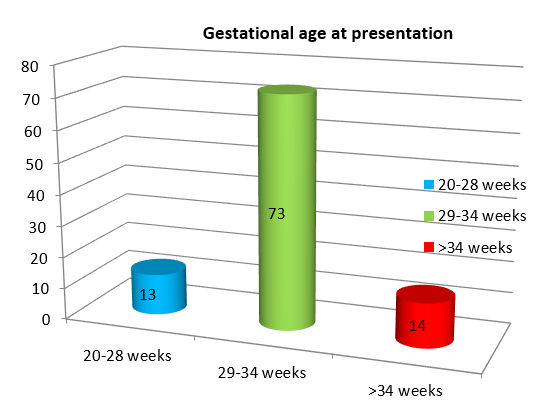
66% of women belonged to lower socio economic status while 29% belonged to middle class. 95% of the women with severe preeclampsia and eclampsia were unbooked cases. 73% of women presented between 29-34 weeks of gestation and 14% were > 34 weeks gestation. ([Figure 2])
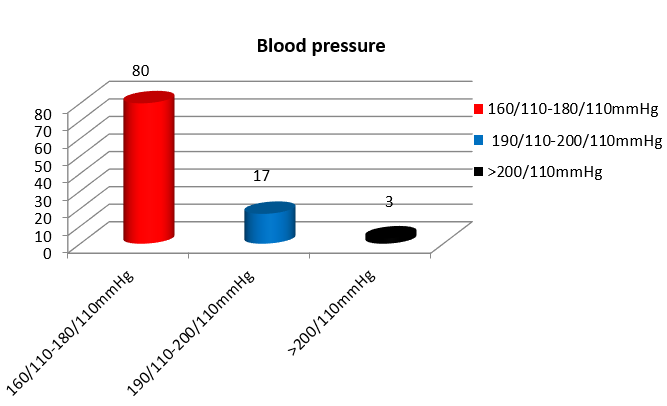
In the study 59% of women were primigravida, 23% were second gravida and 18% were of gravida 3 and above. 80% of women with severe preeclampsia and eclampsia showed blood pressure recordings between 160/110 - 180/110 mmHg. ([Figure 3])
78% of women with severe preeclampsia were given intravenous labetalol only, with 22% required both intravenous labetalol and MgSO4 at the time of presentation due to brisk knee jerks and eclampsia for control of blood pressure.
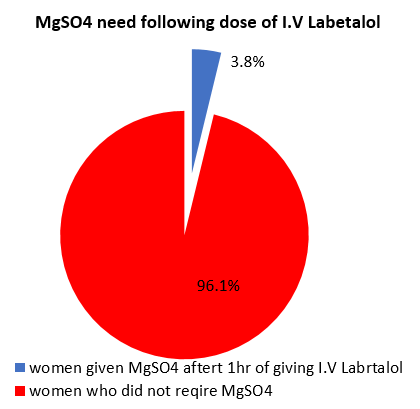
96.1% of the women with blood pressure >160/110 mmHg required only labetalol. 3.8% were given MgSO4 after giving intravenous labetalol due to presence of imminent signs. ([Figure 4])
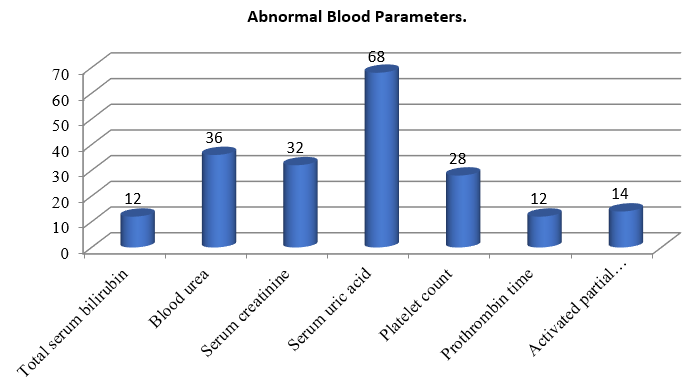
In my study 68% of women in my study had elevated serum uric acid and 36% had raised serum urea. Abnormal platelet count seen in 28% and 32% has raised serum creatinine value. Raised serum bilirubin and PT value seen in 12% and raised APTT seen in 14% cases. ([Figure 5])
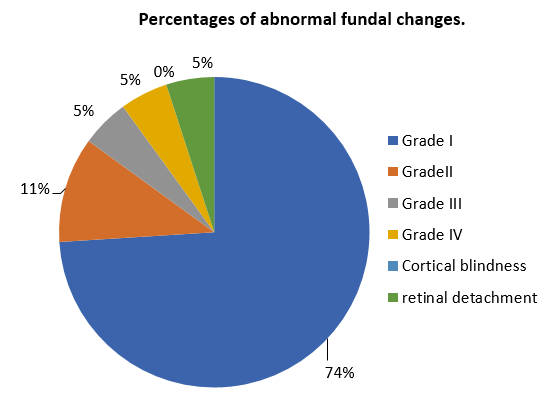
Fundoscopy was done for all the patients to reveal the degree of changes and 19% of women had abnormal fundus. 74% of women showed Grade I fundal changes and 5% had retinal detachment. ([Figure 6])
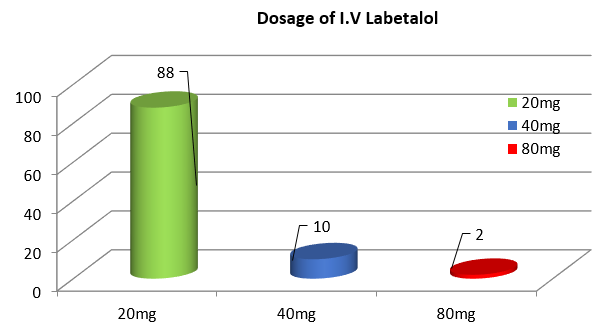
88% of women in my study had blood pressure controlled <160/110mm Hg with a single dose of 20mg intravenous labetalol and 2% required 3 doses. ([Figure 7])
Test for association between I.V Labetolol and the reduction of systolic blood pressure at 10 minutes
Paired T test value- 17.9 pvalue<0.001(highly significant)
Among 100 severe preeclampsia pregnant women, the mean systolic blood pressure before giving I.V Labetolol is 178.6 and the mean systolic blood pressure after 10 minutes of giving i.v Labetolol is 150.1. The mean difference of 28.5 is found to be highly significant.
Test for association between I.V Labetolol and the reduction of diastolic blood pressure at 10 minutes:
Paired T test value-15.63 p value<0.001(highly significant)
Among 100 severe preeclampsia pregnant women, the mean diastolic blood pressure before giving I.V Labetolol 112.9 and the mean diastolic blood pressure after 10 minutes of giving i.v Labetolol is 95.6. The mean difference of 17.3 is found to be highly significant.
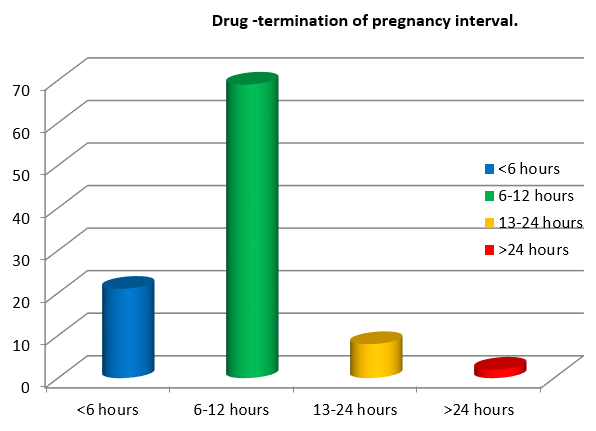
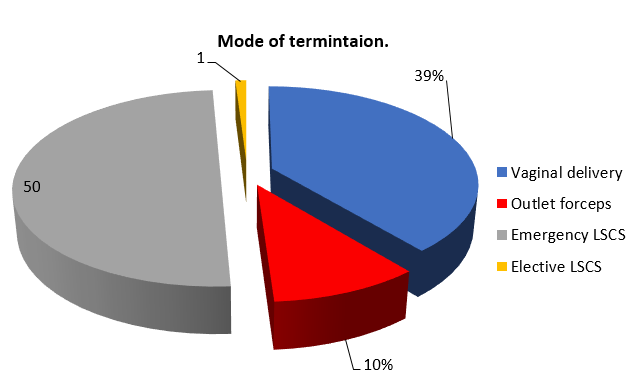
69% of women were delivered at an interval of 6-12 hours after giving Intravenous Labetalol. ([Figure 8])
50% of pregnancies in my study were terminated by Emergency Lower Segment Cesarean Section (LSCS).([Figure 8])
Perinatal outcome
|
APGAR Score |
No. of neonates |
Requiring NICU admission |
Neonates recovered. |
|
8-10 |
38 |
0 |
0 |
|
5-8 |
38 |
15 |
12 |
|
<5 |
9 |
9 |
5 |
|
Birth weight |
No. of neonates |
Percentage |
|
<2 kg |
38 |
38% |
|
2-2.5 kg |
40 |
40% |
|
>2.5 kg |
22 |
22% |
There are 4 neonates in my study who developed hypoglcycemia within 6 hours of life, but all 4 were IUGR babies. Number of still borns and intra uterine death - 15(due to severe preeclampsia and its complications)
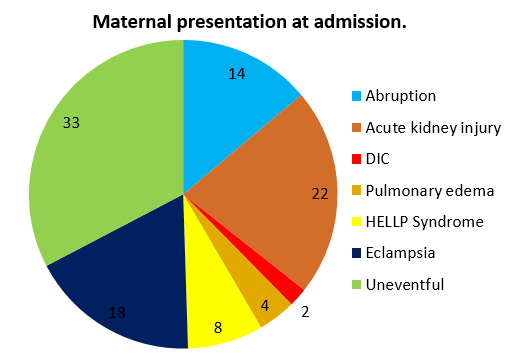
Following intravenous Labetalol only 1.2% developed eclampsia. 22 % of women in my study had acute kidney injury. ([Figure 10])
Discussion
In the present study maximum age incidence was between 20-25 years age group-57%. In the present study 59% of women were primigravidae, which exceeds the results of C.A Micheal [7] which shows 39% incidence of severe preeclampsia, as there are many a number of patients suffering from chronic hypertension than preeclampsia in Australia. In the present study 79% of women had severe preeclampsia and 21% had eclampsia. In the study group 44% belonged to lower socio-economic group. In maximum cases gestational age is between 29-34 weeks gestation. As most of the patients belonged to lower socioeconomic group who do not seek proper antenatal care they presented in advanced stages with complications at an early gestational age.
In this study labetalol lowered the blood pressure in a more predictable manner, without causing sudden hypotension and without effecting utero placental circulation, maternal prostonoids and fetal blood flow as stated by Jouppila Kirkinen et al. [8] In my study, 86 % achieved target blood pressure less < 150/110 mmHg in response to single dose of 20mg I.V labetalol, 12% required 2 doses (20 mg initially followed by 40 mg after 10 minutes), 3 patients required 3 doses (20mg initially followed by 40mg after 10 minutes and then 80 mg after another 10 minutes), while a study conducted in Kaula Lumpur Malaysia , the dose of intravenous labetalol to achieve the target blood pressure was 80mg (3 doses). [9] The difference in the requirement might be due to difference in body mass index. Most of the patients responded to a single dose of 20 mg single dose. 99% of patients in my study required 1 cycle of I.V Labetalol, while only 1 patient require 2 cycles.
LAMPET - Labetelol versus Magnesium sulphate for the Prevention of Eclampsia Trial is a global multicentre randomized control trial was initiated to compare the anti-seizure effect of parenteral MgSO4 versus intravenous labetalol in hypertensive pregnant women who are eligible for MgSO4 therapy. The primary outcome measure is Eclampsia, and the secondary outcome measures include blood pressure control, and relevant antenatal, intrapartum, and postnatal maternal and neonatal parameters including adverse effects and complications.
In my study 78 severe preeclapmtic women were given only Intravenous Labetalol, and among them only 1 patient (1.2%) developed eclampsia, and 77 (98.7%) severe preeclamptic women were prevented from landing into eclampsia and hence avoiding the need of Magnesium sulphate.[10]
Neonates with APGAR score <5 were 9 of which 5 babies recovered and 4 babies died. Based on birth weight, there are 22% babies with weight > 2.5kg, 40% babies with birth weight between 2-2.5 kg, 38% had birth weight <2kg (including still born and IUD). Neonatal hypoglycaemia was seen in 4 IUGR babies and cause was not attributable to labetalol and related to IUGR, which was also documented in other studies. [11], [12]
Therefore by giving a drug which is easily administered, safe, alternative to magnesium sulphate is welcomed and would provide an extremely useful alternative therapy to the current standard of care. If reduction of CPP is an important part of the anti-seizure prophylactic mechanism of action of magnesium sulphate in preeclampsia, then the use of a more specific, less toxic agent may greatly simplify the management of severely preeclamptic women, while at the same time providing equivalent or improved efficacy.
In addition, the facility of administration, reduced risk of respiratory and cardiac depression , lack of need for intensive monitoring of blood magnesium levels, suitability for use by primary care personnel, safety of the regimen, give labetalol very attractive risk/benefit and cost/benefit ratios. We can avoid the need of giving MgSO4 therapy, which is difficult to monitor and is associated with more toxicity which is in accordance with Micheal Belfort study [13] Some authors have therorized that antihypertensive medication alone labetalol or hydralazine [14] is equally efficacious like magnesium sulphate in the prevention of seizure activity. Moodley et al. [15] claimed no additional benefit of magnesium sulphate over antihypertensive medication alone in their study of 228 patients, of whom 112 received both antihypertensive therapy and magnesium sulphate and 116 received antihypertensive therapy only.
Conclusion
Labetalol is more reliable and effective in reducing Cerebral Perfusion Pressure (CPP) in patients with severe preeclampsia. Intravenous labetalol is available as an easily administered, safe alternative to MgSO4 and proven to be a useful alternative therapy to current standard of care. Labetalol has improved efficacy to MgSO4 with lack for intensive monitoring of blood magnesium levels and suitability for use by nursing staff and doctors. The study proves it is devoid of maternal and foetal side effects with good perinatal outcome.
Source of Funding
None.
Conflict of Interest
None.
References
- Berg C, Chang J, Callaghan W, Whitehead S. Pregnancy -related mortality in the United States 1991-1997. Obstet Gynecol. 2003;101(2):289-96. [Google Scholar]
- Spong C, Cunningham F, Leveno K, Bloom S, Hauth J, Rouse D. . Williams Obstetrics. . [Google Scholar]
- Davison J, Baylis C, Swiet M. Medical disorders in obstetric practice. Renal disease. 2008. [Google Scholar]
- Martin J, Hamilton B, Sutton P. Births: Final Data for 2004. Natl Vital Stat Rep. 2004;55(1):1-104. [Google Scholar]
- Zwieten Pv. An overview of the pharmacodynamic properties and therapeutic potential of combined alpha- and beta-adrenoceptor antagonists. Drugs. 1993;45(4):509-17. [Google Scholar]
- EE, Okobi O, Beeko M, Abanga R, Abah N, Briggs L. Comparing Intravenous Labetalol and Intravenous Hydralazine for Managing Severe Gestational Hypertension. Cureus. 2023;15(7). [Google Scholar] [Crossref]
- Michael C. The evaluation of labetalol in the treatment of hypertension complicating pregnancy. Br J Pharmac Suppl. 1982;8(1 Suppl):211-5. [Google Scholar]
- Jouppila P, Kirkinen P, Koivula A, Ylikorkala O. Labetalol does not alter the placental and fetal blood flow or maternal prostanoids in pre-eclampsia. Br J Obstet Gynaecol. 1986;93(6):543-7. [Google Scholar]
- Raheem I, Saaid R, Omar S, Tan P. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomised trial. BJOG. 2012;119(1):78-85. [Google Scholar]
- Kausar M, SH, Hussain R. Comparison of efficacy of intravenous labetalol and intravenous hydralazine for management of pre-eclampsia in pregnant women. Afr Health Sci. 2003;23(1):320-5. [Google Scholar]
- Macpherson M, Pipkin F, Rutter N. The effect of maternal labetalol on the newborn infant. Br J Obstet Gynaecol. 1986;93(6):539-42. [Google Scholar]
- PP, Breart G, Maillard F, Papiernik E, Relier J. The Labetalol Methyldopa Study Group-Comparision of antihypertensive efficacy and perinatal safety of labetalol and methyldopa in the treatment of hypertension in pregnancy: randomized controlled clinical trial. Br J Obstet Gynaecol. 1988;95(9):868-76. [Google Scholar]
- Belfort M, Saade G, Miller CT-, Norwitz E, Nisell H, Grunewald C. Labetalol is more effective and reliable than MgSO4 in reducing maternal cerebral perfusion pressure in severely -preeclamptic women. AJOG. 2001;185(6). [Google Scholar] [Crossref]
- Walker J. Hypertensive drugs in pregnancy. Antihypertension therapy in pregnancy, preeclampsia, and eclampsia. Clin Perinatol. 1991;18(4):845-73. [Google Scholar]
- Moodley J, Moodley V. Prophylactic Anticonvulsant Therapy in Hypertensive Crises of Pregnancy—The Need for a Large, Randomized Trial. Hypertens Pregnancy. 1994;13(3):245-52. [Google Scholar]
- Abstract
- Introduction
- Aims and Objectives
- Materials and Methods
- Results
- Test for association between I.V Labetolol and the reduction of systolic blood pressure at 10 minutes
- Test for association between I.V Labetolol and the reduction of diastolic blood pressure at 10 minutes:
- Perinatal outcome
- Discussion
- Conclusion
- Source of Funding
- Conflict of Interest
- References
Article Metrics
- Visibility 8 Views
- Downloads 3 Views
- DOI 10.18231/pjms.v.15.i.1.93-98
-
CrossMark
- Citation
- Received Date February 28, 2024
- Accepted Date April 08, 2024
- Publication Date March 12, 2025