Panacea Journal of Medical Sciences
Panacea Journal of Medical Sciences (PJMS) open access, peer-reviewed triannually journal publishing since 2011 and is published under auspices of the “NKP Salve Institute of Medical Sciences and Research Centre”. With the aim of faster and better dissemination of knowledge, we will be publishing the article ‘Ahead of Print’ immediately on acceptance. In addition, the journal would allow free access (Open Access) to its contents, which is likely to attract more readers and citations to articles published in PJMS.Manuscripts must be prepared in accordance with “Uniform requiremen...

Evaluation of lung function and oxygen desaturation using spirometry DLCO and 6mwt of hospitalized covid patients on follow up after 1 – 3 months
Abstract
Background: COVID-19, caused by SARS-CoV-2, profoundly impacts the lungs, leading to potential long-term consequences such as fibroproliferation and lung fibrosis.
Aim and Objective: To evaluate post-COVID lung indices, functional capacity, and oxygen desaturation using spirometry, DLCO, and the six-minute walk test (6MWT) through rigorous examination of these parameters.
Materials and Methods: The study employed an observational design, investigating the 1 – 3 months post-COVID health status of 70 hospitalized individuals in a Navi Mumbai tertiary care setting. Ethical approval was secured, and participants provided written informed consent. Assessment procedures included spirometry, DLCO measurement, 6MWT, and CTSS for lung involvement severity. The study protocol adhered to ATS guidelines, capturing comprehensive demographic and COVID-19 admission details. Statistical analysis utilized descriptive statistics due to the absence of specific hypotheses, ensuring rigorous data management and validation.
Results: The study population predominantly comprised individuals aged over 60 years (30%), with a higher representation of males (72.9%) and a majority classified as overweight (51.4%). CORADS scores of 5 (37.1%) were common on HRCT THORAX and higher inflammatory markers (Mean D- Dimer 570.63 and CRP 19.17) during hospitalization. The mean age and BMI were 53.10 ± 13.3 years and 26.34 ± 3.8, respectively. Hypertension (14.29%) was the most prevalent comorbidity. Mean values for CTSS(10.16 ± 5.4), follow-up duration, pulmonary function tests (Mean FVC % was 72.21 ± 14.85 , FEV1 % was 73.84 ± 15.75, DLCO % 63.10 ± 18.04 and RV/TLC % 91.31 ± 21.69 with 17% patients having increased RV/TLC, and biomarkers and oxygen desaturation in 45.7% during the 6-minute walk test.
Conclusion: COVID-19 survivors face lasting lung impairment and exercise limitations post-discharge, emphasizing the need for monitoring and early enrolment in pulmonary rehabilitation programme.
Introduction
Coronavirus Disease 2019 (COVID-19) has emerged as a notable infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).[1] Recent studies have underscored the profound impact of COVID-19 on the lungs, manifesting in various pathophysiological events such as diffuse alveolar epithelium destruction, hyaline membrane formation, capillary damage and bleeding, alveolar septal fibrous proliferation, and pulmonary consolidation. COVID-19 exhibits a distinctive feature of extensive injury to alveolar epithelial and endothelial cells, with consequential fibroproliferation hinting at the potential for chronic vascular and alveolar remodelling that may lead to lung fibrosis or pulmonary hypertension. [2] It raises pertinent concerns regarding the assessment of lung injury in discharged patients.
Various objective assessments of respiratory function are available, with pulmonary function tests (PFTs), including spirometry, diffusion capacity, and lung volumes, being among the most commonly employed. Additional assessments, such as examining respiratory muscles and analyzing airway resistance, offer valuable insights into lung characteristics. These investigations contribute to a holistic comprehension of the implications of acute and chronic respiratory diseases. [3]
Spirometry and DLCO measurements offer insights into lung function indices, particularly regarding diffusion impairment. The six-minute walk test (6MWT) is a simple, objective, and reproducible measure of lung functional capacity. In this test, individuals are instructed to walk as far as possible in six minutes, with the primary outcomes being the six-minute walk distance (6MWD) and Oxygen desaturation. These parameters are often utilized as predictive tools for morbidity or mortality. They are frequently employed to assess the functional status of patients with respiratory and cardiovascular diseases before and after interventions or specific treatments. [4]
Severe COVID-19 cases demonstrate a higher incidence of impaired diffusion capacity of the lung for carbon monoxide (DLCO) and experience more significant decreases in total lung capacity (TLC) and declines in six-minute walk distance (6MWD) compared to non-severe cases at 30 days post-discharge. However, clinical evidence for the long-term follow-up of pulmonary function and physiological disorders in severe COVID-19 patients is currently lacking. [5]
In the Indian population, studies are scarce, and data remains limited, especially concerning post-COVID patients. It underscores the importance of further research to comprehensively understand the implications of COVID-19 on lung function, particularly in the long term, and establish effective post-discharge care strategies. [6]
The primary objective of our study is to evaluate post-COVID lung indices, functional capacity, and oxygen desaturation using spirometry, DLCO, and the six-minute walk test (6MWT) through rigorous examination of these parameters. This objective will enhance our understanding of post-COVID lung health and aid in developing targeted interventions and management strategies for individuals in the recovery phase.
Materials and Methods
Study design and duration
The research employed a prospective observational design, conducted over two years, to investigate the post-COVID health status of individuals in a tertiary care setting in Navi Mumbai. The study was meticulously conducted within the Tertiary care setting, Navi Mumbai. Institutional Ethics Committee approval was secured before initiation, and each subject underwent a careful written informed consent process before enrolment. A targeted sample size of 70, post-COVID patients was selected based on specific criteria.
Inclusion criteria
In this study all individuals undergoing follow-up post-COVID between 30 to 100 days post COVID infection who willingly provided consent are included.
Exclusion criteria
In this study non-consenting individuals, those with physical disabilities or comorbidities precluding pulmonary function testing, and individuals suspected but not diagnosed or treated for COVID-19 were excluded from the study.
Study procedure
A detailed history of the patients regarding their course during hospitalization whether in ward or in ICU, presence of co-morbidities and demographic profile was taken. Biochemical Investigations and Biomarkers including D- Dimer and CRP levels, CORADS and CT Severity Score, CORADS and CT Severity Score based on HRCT Thorax done during hospitalisation were determined. The comprehensive assessment involved spirometry, measurement of the diffusing capacity of lungs for carbon monoxide (DLCO), and the 6-minute walk test (6MWT) for all 70 post-COVID patients. Key lung function indices were meticulously evaluated, including FEV1, FVC, FEV1/FVC ratio, DLCO, KCO (Carbon Monoxide transfer coefficient), and RV/TLC ratio. 6MWT parameters, such as 6-minute walking distance (6MWD), minimum and maximum heart rate (HR), baseline and end HR, as well as oxygen saturation (SpO2) and blood pressure, were recorded.
CTSS (CT severity score)
The severity of lung involvement was assessed through the CT Severity Score (CTSS) (with a maximum score of 25), based on the presence of multifocal Ground Glass Opacities in each lobe on HRCT Thorax, scored according to the assessment of a radiologist.
Study protocol highlights
Spirometry, DLCO, and the 6-Minute Walk Test adhered to the American Thoracic Society (ATS) guidelines, ensuring standardized and consistent assessments. The comprehensive study protocol aimed to capture a holistic understanding of the post-COVID health status among the participants.
Statistical analysis
Rigorous data management was implemented, involving recording information in an Excel spreadsheet and thorough validation to identify and rectify inconsistencies and errors. Given the absence of specific study hypotheses, the analysis primarily utilized descriptive statistics, including percentages, frequencies, mean, and standard deviation.
Results
|
Age group |
Frequency (N) |
Percentage (%) |
|
24 to 40 years |
13 |
18.6 |
|
41 to 50 years |
18 |
25.7 |
|
51 to 60 years |
18 |
25.7 |
|
more than 60 years |
21 |
30 |
|
Gender |
||
|
Female |
19 |
27.1 |
|
Male |
51 |
72.9 |
|
BMI |
||
|
Normal |
22 |
31.4 |
|
Obese |
12 |
17.1 |
|
Overweight |
36 |
51.4 |
|
CORADS |
||
|
0 |
2 |
2.9 |
|
1 |
5 |
7.1 |
|
2 |
7 |
10 |
|
3 |
9 |
12.9 |
|
4 |
9 |
12.9 |
|
5 |
26 |
37.1 |
|
6 |
12 |
17.1 |
|
Values are expressed as frequency and percentage. |
[Table 1] shows that most of the study population belongs to the age group of more than 60 years (30%), followed by 51 to 60 years (25.7%) and 41 to 50 years (25.7%). There was male predominance (72.9%) amongst the study population compared to females (27.1%). Most of the study population was Overweight (51.4%), followed by normal (31.4%) and obese (17.1%). The study population had a CORADS score of 5 (37.1%) followed by a CORADS score of 6 (17.1%) and CORADS score of 3 & 4 (12.9 each) on HRCT THORAX done during hospitalization for COVID-19. The mean age and BMI were 53.10 ± 13.3 years and 26.34 ± 3.8, respectively.
|
Ward/ICU |
Frequency (N) |
Percentage (%) |
|
ICU |
21 |
30 |
|
Ward |
49 |
70 |
|
O2 Requirement |
||
|
No |
41 |
58.6 |
|
Yes |
29 |
41.4 |
|
PFT abnormalities |
||
|
Mixed defect |
1 |
1.4 |
|
Normal |
20 |
28.6 |
|
Obstructive airway disease |
9 |
12.9 |
|
Restrictive airway disease |
40 |
57.1 |
|
Values are expressed as frequency and percentage. |
Hypertension (14.29%) was the most common Comorbidities amongst the study population, followed by Bronchial Asthma (7.14%), DM (5.71 %) and IHD (4.28%) shown in [Figure 1].
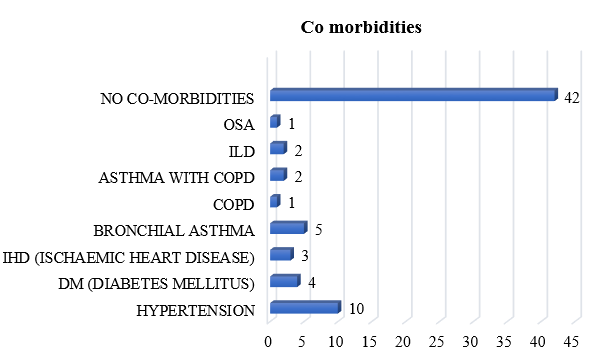
70% of the study population were admitted to the ward, while 30% were admitted to ICU during COVID-19 Hospitalization. O2 Requirement during COVID-19 hospitalization was observed in 41.4% of the study population. Restrictive airway disease (57.1%) was the most common PFT abnormality amongst the study population, followed by Obstructive airway disease (12.9%) and Mixed defect (1.4%), shown in [Table 2].
|
SpO2 |
Mean |
Std. Deviation |
|
Baseline |
97.13 |
1.2 |
|
Min spo2 |
94.11 |
2.8 |
|
Max spo2 |
97.5 |
1.2 |
|
End |
95.44 |
1.9 |
|
Mean spo2 |
95.03 |
2 |
|
6MWT |
||
|
Actual Distance Walked |
367.43 |
83.6 |
|
Predicted Distance |
548.51 |
77.5 |
|
% predicted |
67.11 |
11.6 |
|
Heart rate |
||
|
Baseline HR |
93 |
12.1 |
|
Min HR |
87.94 |
9.63 |
|
Mean |
113.61 |
12.91 |
|
End HR |
106.47 |
16.15 |
|
Max HR |
118.31 |
19.17 |
|
Values are expressed in Mean and Standard Deviation. |
In [Table 3], Baseline SpO2, minimum SpO2, maximum SpO2, End and Mean SpO2 on 6 Minute Walk Test were 97.13 ± 1.2, 94.11 ± 2.8, 97.50 ± 1.2, 95.44 ± 1.9 and 95.03 ± 2 respectively. in 6-minute walk test, actual distance walked, Predicted distance and % predicted was 367.43 ± 83.6, 548.51 ± 77.5 and 67.11 ± 11.6 respectively. Baseline Heart rate, minimum Heart rate, maximum Heart rate, End and Mean Heart rate were 93.00± 12.10, 87.94 ± 9.63, 118.31 ± 19.17, 106.47 ± 16.15and 113.61 ± 12.91, respectively.
|
|
Mean |
Std. Deviation |
|
CTSS (CT severity score) |
10.16 |
5.4 |
|
Follow up |
61.7 |
21.9 |
|
PFT |
||
|
FVC % |
72.17 |
14.86 |
|
FEV1 % |
73.77 |
15.81 |
|
FEV1/FVC % |
82.94 |
9.57 |
|
DLCO % |
63.04 |
18 |
|
KCO |
2.935 |
1.09 |
|
RV/TLC % |
91.31 |
21.69 |
|
RV/TLC % pred |
121.87 |
18.35 |
|
Biomarkers |
||
|
D Dimer |
570.63 |
493.12 |
|
CRP |
19.17 |
22.59 |
|
Values are expressed in Mean and Standard Deviation. |
[Table 4] shows the Mean and Standard deviation of CTSS and Follow-up was 10.16 ± 5.4 and 61.70 ± 21.9, respectively. Mean FVC %, FEV1 %, FEV1/FVC %, DLCO %, KCO, RV/TLC % and RV/TLC % pred was 72.17 ± 14.86, 73.77 ± 15.81, 82.94 ± 9.57, 63.04 ± 18.00, 2.935 ± 1.090, 91.31 ± 21.69 and 121.87 ± 18.35 respectively. mean D Dimer and CRP was 570.63 ± 493.12 and 19.17 ± 22.59, respectively.
Discussion
The current study aimed to comprehensively evaluate pulmonary function, gas exchange abnormalities, and exercise limitations in COVID-19 survivors nearly 2 months after hospital discharge. In agreement with previous studies on SARS and MERS epidemics, [7] we found evidence of persistent lung impairment and disability in many COVID-19 patients (71.4 % patients).
Pulmonary function tests revealed restrictive ventilatory defects in 57.1 % of the patients, with mean FVC and FEV1 approximately 70% of predicted. While airway obstruction was not observed based on preserved FEV1/FVC ratios, the substantially reduced DLCO (63% predicted) confirms parenchymal dysfunction and impaired gas exchange.[8] Our findings parallel those of Mo et al. and Huang et al., who also reported persistent restriction following COVID-19 pneumonia.[2], [5] However, the severity of impairment was greater in our cohort, likely reflecting differences in illness severity given 30% of ICU admissions.
In keeping with the restrictive physiology, we provide initial evidence of gas trapping on lung volume assessment. The increased RV/TLC ratio in 17% of the patients indicates the involvement of the larger airways from residual cellular inflammation rather than small airway disease. This metric has not been previously investigated in the post-COVID-19 setting by George et al.[9]
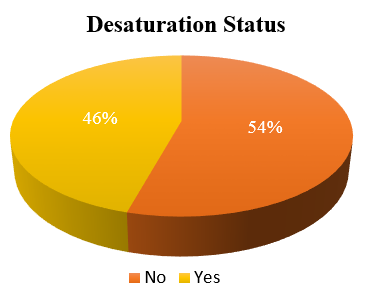
Alongside the abnormalities in static lung volumes and diffusion capacity, we also observed significant exertional oxygen desaturation in 45.7% of patients on the six-minute walk test (Figure 2). The distance walked was also substantially reduced compared to healthy predictions. Our findings align with previous reports confirming impaired exercise capacity and dynamic gas exchange abnormalities after COVID-19. [10]
These quantitative lung function and exercise test findings likely arise from parenchymal scarring and fibrosis triggered by COVID-19 pneumonia. The initial severe inflammatory response causes diffuse alveolar damage, activation of myofibroblasts, and extracellular matrix deposition.[11] Persistence of these histopathological changes despite radiographic and clinical recovery can lead to restrictive ventilatory changes, reduced gas transfer efficiency, expiratory flow limitation, and dynamic hyperinflation - all observed in the present study.[12]
We found more pronounced physiological impairments compared to prior post-COVID-19 literature, which likely relates to differences in cohort illness severity. Our study comprised older patients with high rates of ICU admissions, oxygen requirements, and radiological injury based on CT severity scores. Previous autopsy and radiological descriptions suggest extensive pulmonary fibrosis in severe COVID-19 cases.[13], [14] Hence, the magnitude of the initial inflammatory insult likely underlies the extent of permanent parenchymal scarring and physiological dysfunction.[15] Close monitoring of lung function and exercise capacity is warranted in severe post-COVID-19 patients to guide supportive therapies.
Strengths of the present study include the comprehensive panel of physiological tests performed and analysis of key predictors of persistent disability like illness severity, oxygen needs, and radiologic scores. However, longer-term follow-up is required to definitively characterize the natural history of post-COVID-19 lung disease over time. Serial physiological and radiographic surveillance will clarify recovery trajectories and enable testing of novel anti-inflammatory and antifibrotic agents.[9] Our findings add to the emerging literature on post-COVID-19 syndrome, highlighting the pulmonary morbidities seen in severe illness survivors.
Conclusion
The current study demonstrates that COVID-19 survivors experience persistent lung function impairment and reduced exercise tolerance in the 2 months following hospital discharge. Specifically, over half of the patients exhibited restrictive defects and diminished diffusion capacity and gas exchange efficiency. Additionally, a significant proportion was desaturated during the six-minute walk test, confirming exertional oxygen impairment.
These findings underline the importance of close monitoring and pulmonary rehabilitation for post-COVID-19 patients. Tailored rehabilitative strategies can help mitigate persistent respiratory disability through assessment, active exercise training, patient education, nutritional support, and psychosocial care. Early integration of pulmonary rehabilitation is advocated in the post-acute recovery phase to optimize long-term outcomes for COVID-19 survivors. However, longer-term studies are warranted to characterize this population's physiological recovery trajectories.
Ethical Consideration
Ethical approval was diligently obtained from the institutional ethics committee before initiating the study, ensuring compliance with established ethical standards.
Informed Consent
A detailed written informed consent process was followed for each participant, emphasizing transparency and comprehension. An informative sheet was distributed to all participating patients, ensuring clarity regarding the study's nature and objectives.
Source of Funding
None.
Conflict of Interest
None.
References
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565-74. [Google Scholar]
- Mo X, Jian W, Su Z, Chen M, Peng H, Peng P. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55(6). [Google Scholar] [Crossref]
- Gold W, Koth L. Pulmonary Function Testing. Murray Nadel's Textbook Respir Med. 2023. [Google Scholar] [Crossref]
- Zarogoulidis P, Kerenidi T, Huang H, Kontakiotis T, Tremma O, Porpodis K. Six minute walking test and carbon monoxide diffusing capacity for non-small cell lung cancer: easy performed tests in every day practice. J Thorac Dis. 2012;4(6):569-76. [Google Scholar]
- Huang Y, Tan C, Wu J, Chen M, Wang Z, Luo L. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1). [Google Scholar] [Crossref]
- Lakshmanan S, Mv B, Dharmalingam A, Bhaskar E, Anil A, Senthil N. Post-acute Sequelae of SARS-CoV-2 Infection: Do Indians Fare Better?. Cureus. 2022;14(12). [Google Scholar] [Crossref]
- Zhao Y, Shang Y, Song W, Li Q, Xie H, Xu Q. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25. [Google Scholar] [Crossref]
- Pereira M, Mohan S, Cohen D, Husain S, Dube G, Ratner L. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800-8. [Google Scholar]
- George P, Wells A, Jenkins R. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807-15. [Google Scholar]
- Swarnakar R, Yadav S. Rehabilitation in long COVID-19: A mini-review. World J Methodol. 2022;12(4):235-45. [Google Scholar]
- Borg K, Stam H. Rehabilitation of post-Covid - 19 syndrome - once again a call for action!. J Rehabil Med. 2021;53(1). [Google Scholar] [Crossref]
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-32. [Google Scholar]
- Wichmann D, Sperhake J, Lütgehetmann M, Steurer S, Edler C, Heinemann A. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173(4):268-77. [Google Scholar]
- Pan F, Ye T, Sun P, Gui S, Liang B, Li L. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295(3):715-21. [Google Scholar]
- Chan K, Zheng J, Mok Y, Li Y, Liu Y, Chu C. SARS: prognosis, outcome and sequelae. Respirology. 2003;8(1):36-40. [Google Scholar]
- Abstract
- Introduction
- Materials and Methods
- Study design and duration
- Inclusion criteria
- Exclusion criteria
- Study procedure
- CTSS (CT severity score)
- Study protocol highlights
- Statistical analysis
- Results
- Discussion
- Conclusion
- Ethical Consideration
- Informed Consent
- Source of Funding
- Conflict of Interest
- References
Article Metrics
- Visibility 7 Views
- Downloads 2 Views
- DOI 10.18231/pjms.v.15.i.1.122-127
-
CrossMark
- Citation
- Received Date December 08, 2023
- Accepted Date May 29, 2024
- Publication Date March 12, 2025