Panacea Journal of Medical Sciences
Panacea Journal of Medical Sciences (PJMS) open access, peer-reviewed triannually journal publishing since 2011 and is published under auspices of the “NKP Salve Institute of Medical Sciences and Research Centre”. With the aim of faster and better dissemination of knowledge, we will be publishing the article ‘Ahead of Print’ immediately on acceptance. In addition, the journal would allow free access (Open Access) to its contents, which is likely to attract more readers and citations to articles published in PJMS.Manuscripts must be prepared in accordance with “Uniform requiremen...

Histopathological study of salivary gland lesions in a tertiary care hospital of Central India
Abstract
Introduction: Less than 1% of all malignancies and 3-5.5% of all head and neck tumors are salivary gland neoplasms. Salivary gland tumors occur in 0.4 to 13.5 instances per 1 lakh people worldwide each year. Salivary gland neoplasms include a wide range of benign and malignant tumors.
Aim and Objective: This study aims to categorize different salivary gland tumours in accordance with WHO classification and to examine the frequency of salivary gland lesions with relation to age, sex, and location of lesion.
Materials and Methods: Salivary gland soecimens were delivaered to the histopathology section in 10% formalin. They were carefully labelled, fixed and meticulously prepared for gross examination. Sections were taken from the lesion, margins, surrounding tissue and lymph nodes if any. 3-5 micrometer serially cut paraffin wax sections were processed. Hematoxylin and eosin (H&E) stained slides were examined and findings were documented. The acquired data underwent analysis and tabulation utilizing SPSS software.
Results: 64 specimens of salivary gland lesions were studied in the present study. The age range of these patients varied from 13 years to 76 years. Benign cases formed the bulk of this study (54.69%) followed by non- neoplastic and malignant cases. Benign tumors were predominantly noted in the neoplastic lesions studied accounting for 72.92% cases followed by malignant tumors 27.08%. Pleomorphic adenoma was the only benign neoplasm encountered in our study.
Conclusion: The most predominant malignant neoplasm was mucoepidermoid carcinoma. Adenoid cystic carcinoma, Secretory carcinoma, Clear cell carcinoma and Polymorphous adenocarcinoma were also reported.
Introduction
Salivary glands are exocrine glands that secrete saliva and aid in the process of food digestion. The lubricating effect of saliva makes articulation and chewing easy and comfortable. It also decreases the accumulation of bacteria in intraoral structures, hence decreasing the chances of infection.[1]
Salivary glands are situated in the lips, cheeks, palate, and tongue, among other areas of the oral cavity. They have been divided into three pairs of main glands: Submandibular, sublingual, and parotid.[2]
Less than 1% of all malignancies and 3-5.5% of all head and neck tumors are salivary gland neoplasms. Salivary gland tumours occur in 0.4 to 13.5 instances per 1 lakh people worldwide each year. A variety of benign and malignant tumors can be classified as salivary gland neoplasms. These tumours may provide diagnostic issues because of their extensive morphological extent, histological overlap across numerous cancer categories, and uncommon occurrence.[3]
About 80% of salivary gland cancers are found in parotid glands, with 10% to 15% occurring in submandibular glands. Pleomorphic adenoma constitutes 70% of all benign tumours.[4]
The nonneoplastic entities include a wide range of inflammatory conditions such as infectious, granulomatous or autoimmune aetiologies as well as obstructive, developmental and idiopathic disorders. These often present clinically as tumours and may have pathological feature similar to some of the neoplasms.[5]
Aims and Objectives
This study aims to categorize different salivary gland tumours in accordance with WHO classification and to examine the frequency of salivary gland lesions with relation to age, sex, and location of lesion.
Materials and Methods
This study was conducted in a tertiary care facility of Nagpur in Central India over a duration of one year. The histopathology section received salivary gland lesions in 10% formalin in appropriately sized containers. Upon arrival, they were carefully identified, fixed, and grossed. Representative tissue bits were excised from the specimen, margins, surrounding tissue and lymph nodes if any. 3-5 micrometer serially cut paraffin wax sections were processed. Hematoxylin and eosin (H and E) stained slides were studied and reported. Data obtained were analyzed and tabulated using SPSS software.
Inclusion criteria
All the patients who presented to the surgery/ ENT departments and diagnosed clinically and radiologically and who are willing to gave consent were included in this study.
Exclusion criteria
The present investigation excluded patient undergoing biopsies who did not willing to gave consent for surgery.
Results
The present study conducted included 64 cases of salivary gland lesions. These patients were in the age range of 13 to 76 years old. Lesions involving the salivary glands ranged in size from 3 x1 cm to 8 x6 cm. With a mean age of 36.83 years, the most number of cases (31.25%) were found in the 31–40 age range. The age range of 71 to 80 years old had the fewest number of cases.
Our study revealed a male predominance in salivary gland lesions with a male: female ratio of 1.46:1 noted in this study. ([Table 1])
Parotid gland (78.13%) was most commonly affected followed by the submandibular gland (20.31%). ([Table 2])
Benign cases formed the bulk of this study (54.69%) followed by non- neoplastic and malignant cases. Out of a total of 64 cases, 25% were non-neoplastic lesions and 75% were neoplastic lesions. Among benign lesions, chronic sialadenitis with sialolithiasis (56.25%) formed the bulk of this study followed by sialadenosis (31.25%). The most frequent non-neoplastic lesion was chronic sialadenitis, which affected 14.06% of the population overall. Sialadenosis, on the other hand, impacted only 7.81% of the population.([Table 3])
Of the neoplastic lesions examined, benign tumors accounted for 72.92% of the cases, whereas malignant tumors represented only 27.08%. Pleomorphic adenoma was the only benign neoplasm encountered in our study. The most predominant malignant neoplasm was mucoepidermoid carcinoma. Adenoid cystic carcinoma, secretory carcinoma, clear cell carcinoma, and polymorphous adenocarcinoma were among the other malignant salivary gland cancers. ([Table 3])
|
|
Parotid gland |
Submandibular gland |
Sublingual gland |
||||||
|
Sex |
Total |
Non neoplastic |
Benign |
Malignant |
Non neoplastic |
Benign |
Malignant |
Benign |
Malignant |
|
Male |
38 |
07 |
21 |
03 |
03 |
02 |
02 |
- |
- |
|
Female |
26 |
04 |
12 |
04 |
02 |
01 |
02 |
- |
01 |
|
Site |
Number of cases |
Percentage (%) |
|
Parotid gland |
51 |
79.69 |
|
Submandibular gland |
12 |
18.75 |
|
Sublingual gland |
01 |
1.56 |
|
Total |
64 |
100 |
|
Salivary gland Lesion |
11-20 |
21-30 |
31-40 |
41-50 |
51-60 |
61-70 |
71-80 |
Total |
|
Chronic sialadenitis with sialolithiasis |
03 |
03 |
|
|
02 |
01 |
|
09 |
|
Sialadenosis |
01 |
|
02 |
02 |
|
|
|
05 |
|
Lymphoepithelial cyst |
01 |
|
01 |
|
|
|
|
02 |
|
Pleomorphic adenoma |
|
12 |
14 |
06 |
03 |
|
|
35 |
|
Mucoepidermoid carcinoma |
|
|
|
02 |
03 |
|
|
05 |
|
Adenoid cystic carcinoma |
|
|
03 |
|
|
|
01 |
04 |
|
Secretory carcinoma |
|
|
|
01 |
01 |
|
|
02 |
|
Clear cell carcinoma |
|
|
|
|
|
01 |
|
01 |
|
Polymorphous adenocarcinoma |
|
|
|
|
01 |
|
|
01 |
Discussion
Salivary glands are located in and around the oral cavity, primarily in the lips, cheeks, palate, and tongue. They are divided into paired primary glands, namely the parotid, submandibular, and sublingual glands. Salivary glands vary in the nature of secretions. The parotid gland secretes serous fluid, submandibular gland secretes mucinous secretion and sublingual gland has seromucinous secretions. The salivary gland is made up of myoepithelial cells, fibrous tissue, blood vessels, nerves, and connective tissue stroma, as well as serous or mucinous acini and ducts. [2]
Salivary gland tumors account for a wide range of benign and malignant tumors, each with its own unique physical architecture, varied clinical features, and an uncertain prognosis are represented by salivary gland tumors. An estimated 0.4–13 salivary gland neoplasms occur in every 100,000 patients annually. [6]
Neoplastic lesions (75%) formed the bulk of this study followed by non-neoplastic (25%) conditions of salivary gland. Benign tumors accounted for 54.687% of all neoplasms followed by malignant tumors (45.312%). Two cases of secretory carcinoma were also noted in our study which were confirmed on immunohistochemistry.
Pleomorphic adenoma was the only benign tumor noted in our study. Out of a total of 35 pleomorphic adenoma cases, majority of (n= 27) were noted in parotid gland and 08 cases were noted in submandibular gland. These findings correlated with studies conducted by Vedula B et al., [7] Jude U et al., [8] Subhashraj et al., [9] Ito FA et al.[10] and Dharaiya C.M et al., [11]
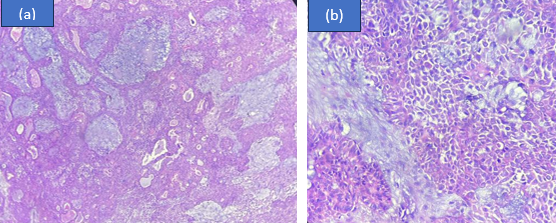
Mucoepidermoid carcinoma was the predominant malignancy noted in our study. (n=5, 38.46%) On gross examination mucoepidermoid carcinoma displays irregular, solid, soft-firm, greyish white tumor. Cut section revealed multiple cystic spaces, largest cyst of size 2.5x1 cm in size.
Three cell types were identified upon microscopic inspection: squamous cells, intermediate cells, and mucin-secreting cells. Sheets and nests of malignant cells infiltrating surrounding stroma was also noted. ([Figure 2]) Due to the variations in cells and architecture of mucoepidermoid carcinoma, extensive grossing was performed to rule out any discrepancy.
Mucoepidermoid carcinomas are categorized as high grade, intermediate grade and low grade malignancies based on atypia.[12]
We documented nine cases of chronic sialadenitis associated with sialolithiasis. Submandibular gland was the most commonly affected. Ultrasonography revealed well defined heterogeneously hypoechoic solid lesions with sialoliths of size ranging from 7-9 mm. Contrast enhancing CT scans revealed dilated submandibular ducts and hyperdense calcific foci.
Chronic sialadenitis is associated with mild clinical symptoms. Sjogrens syndrome also known as keratoconjunctiva sicca manifests itself by involving both lacrimal and salivary glands. Histological features of mild to moderate lymphoplasmacytic infiltration accompanied by varying degrees of atrophy, fibrosis, and microliths are seen in chronic sialadenitis in the presence of obstruction. [13]
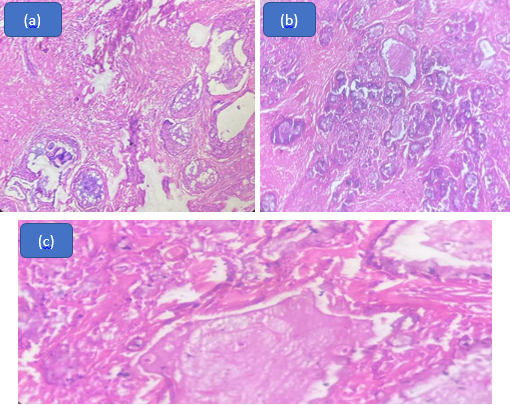
In our analysis, we identified 4 cases of adenoid cystic cancer. On microscopic examination, tumour cells were arranged in a cribriform pattern separated by sharply punched out spaced filled with basophilic matrix. Bilayered tubules were noted with true lumen. Tumor cells had scant cytoplasm with small, hyperchromatic nuclei. ([Figure 3])
Adenoid cystic carcinoma has three histological variants namely a) Cribriform b) Tubular and c) Solid variants. Amongst the three, the cribriform variant is the commonest and solid type is least common. Pure adenoid cystic carcinoma is rarely seen in salivary gland neoplasms, they are usually mixed in nature with each variant contributing to some part of the tumor. However these tumors are classified based on the histologic pattern that predominates. Histological typing is primarily used to determine the prognostic significance of the variants of Adenoid cystic carcinoma. The well differentiated tubular pattern of adenoid cystic carcinoma has the best prognosis as compared to moderately differentiated cribriform pattern and poorly differentiated solid pattern Adenoid cystic carcinomas. [14]
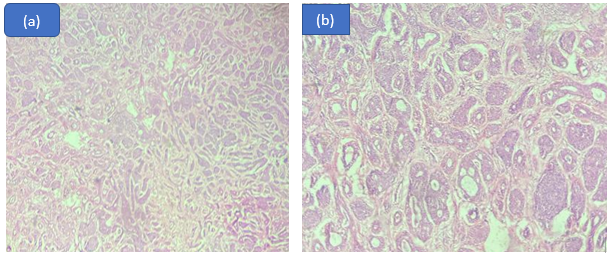
We reported one case of clear cell carcinoma in the sublingual gland of a 64 year old female patient. On examination, patient presented with a painless, firm lump which rapidly increased in size.
CCC is usually located in minor salivary glands of female patients between the ages of 50 and 80. It is slow growing in nature and hardly affects lymph nodes or spreads to distant locations. [15] Clear cell carcinoma was originally referred to as Hyalinising clear cell carcinoma due to its distinctive appearance of nests, islands, and sheets of monomorphic, bland appearing malignant cells with clear cytoplasm in a hyalinized stroma. [16] ([Figure 4])
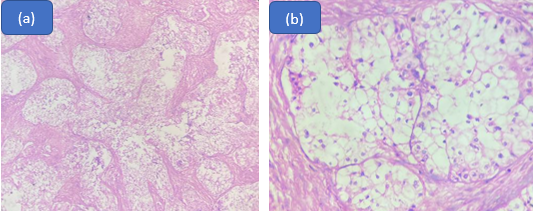
We also reported one case of Polymorphous adenocarcinoma in a 56 year old female.
Salivary gland polymorphous adenocarcinoma (PAC), formerly known as polymorphous low-grade adenocarcinoma (PLGA), is an unusual kind of malignancy. A yearly incidence of 0.051 incidents per 100,000 people has been reported in earlier research. [17] ([Figure 5])
The world health organization recently updated this neoplasm by removing the term low grade due to emergence of evidence showing 19% recurrence rates and risks of transformation into high- grade malignancies. [18]
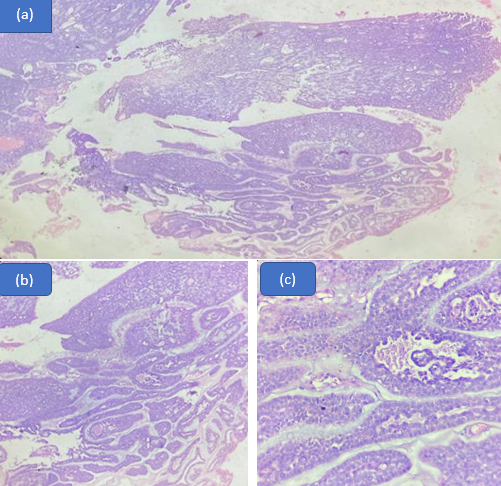
Conclusion
The findings of the present study reveal the variable nature of histomorphological patterns in the wide range of salivary gland tumours. Pleomorphic adenoma and mucoepidermoid carcinoma are the two salivary gland neoplasms that are most frequently seen as benign and malignant neoplasm respectively. Due to the small number of cases of each tumor type that were available for this investigation, some tumour types may not have been included because of their comparatively rare occurrence.
Source of Funding
None.
Conflict of Interest
None.
References
- Pachori G, Chandra S, Bihari NA, Kasliwal N. Histopathological spectrum of salivary gland lesions in Ajmer region. Int J Res Med Sci. 2019;7(7):2708-13. [Google Scholar]
- Geethalakshmi U, Rupashree S, Babu KR. Study of Histopathological Diversity in Salivary Gland Lesions. J Med Sci Health. 2021;7(2):67-73. [Google Scholar]
- Srinivasan G, Joseph F, Kaliyaperumal S, Ponnaiah A. Histopathological spectrum of salivary gland lesions - in a tertiary care centre. Int J Res Med Sci. 2021;9(1):97-101. [Google Scholar]
- Prasad G, Saxena R. Histopathological spectrum of salivary gland lesions in hadoti region. J Diagn Pathol Oncol. 2019;4(4):315-9. [Google Scholar]
- Soni D, Mathur K, AY, Kumar V. Histopathological Spectrum of Salivary Gland Lesions in Tertiary Care Centre at SMS Medical College, Jaipur, Rajasthan. Int J Med Res Prof. 2016;2(2):209-15. [Google Scholar]
- Theresa J, Harke A, Lavanya M. A study on the morphological spectrum of salivary gland tumors. Indian J Pathol Oncol. 2020;7(1):1-4. [Google Scholar]
- Vedula B, Srikanth R, Naidu S, Sudhakar R. Histopathological Study of Salivary Gland Tumours. J Evid Based Med Healthc. 2020;7(18):900-3. [Google Scholar]
- Jude U, Olu-Eddo A. Salivary gland tumors, a twenty-year retrospective study. Afr J Med Health Sci. 2014;13(1). [Google Scholar]
- Subhashraj K. Salivary gland tumors: a single institution experience in India. Br J Oral Maxillofac Surg. 2008;46(8):635-8. [Google Scholar]
- Ito F, Ito K, Vargas P, Almeida Od, Lopes M. Salivary gland tumors in a Brazilian population: a retrospective study of 496 cases. Int J Oral Maxillofac Surg. 2005;34(5):533-6. [Google Scholar]
- Dharaiya CM, PM. Comparative study of Cytodiagnosis of salivary gland neoplasm with histopathology. Trop J Path Micro. 2018;4(1):88-92. [Google Scholar]
- Mills S. Head and neck. In: Sternberg’s Diagnostic Pathology. . 2010. [Google Scholar]
- Wenig B, Carlson D. The head and neck. Essentials of Rubins Pathology. 2014. [Google Scholar]
- Chundru N, Amudala R, Thankappan P, Nagaraju C. Adenoid cystic carcinoma of palate: A case report and review of literature. Dent Res J. 2013;10(2):274-8. [Google Scholar]
- El-Naggar A, Chan J, Grandis J, Takata T, Slootweg P. World Health Organization classification of head and neck tumours. . 2017. [Google Scholar]
- Milchgrub S, Gnepp D, Vuitch F, Delgado R, Albores-Saavedra J. Hyalinizing clear cell carcinoma of salivary gland. Am J Surg Pathol. 1994;18(1):74-82. [Google Scholar]
- XM, Katabi N, McGill M, Hay A, DZ, Shah J. Polymorphous adenocarcinoma of salivary glands. Oral Oncol. 2019;95:52-8. [Google Scholar] [Crossref]
- Kimple A, Austin G, Shah R, Welch C, Funkhouser W, Zanation A. Polymorphous low-grade adenocarcinoma: a case series and determination of recurrence. The Laryngoscope. 2014;124(12):2714-9. [Google Scholar]
Article Metrics
- Visibility 7 Views
- Downloads 4 Views
- DOI 10.18231/pjms.v.15.i.1.143-147
-
CrossMark
- Citation
- Received Date February 16, 2024
- Accepted Date July 01, 2024
- Publication Date March 12, 2025