Panacea Journal of Medical Sciences
Panacea Journal of Medical Sciences (PJMS) open access, peer-reviewed triannually journal publishing since 2011 and is published under auspices of the “NKP Salve Institute of Medical Sciences and Research Centre”. With the aim of faster and better dissemination of knowledge, we will be publishing the article ‘Ahead of Print’ immediately on acceptance. In addition, the journal would allow free access (Open Access) to its contents, which is likely to attract more readers and citations to articles published in PJMS.Manuscripts must be prepared in accordance with “Uniform requiremen...

Splenic abscess in a diabetic patient, Melioidosis: A case study
Abstract
Burkholderia pseudomallei is the causative organism for Melioidosis. This is a ubiquitous organism which produces symptoms ranging from no symptoms to abscess in internal organs like liver and spleen finally leading to septic shock.
45 years old female patient doing agriculture having diabetes and hypertension presented with high grade fever and acute lower abdominal pain. CT abdomen showed multiple well defined lesions involving spleen. USG guided pigtail aspiration of the purulent aspirate in direct Gram stain revealed many pus cells, Gram negative bacilli seen (safety pin appearance). Cultured on standard bacteriological media, identified as Burkholderia pseudomallei with conventional techniques.
Chronic case of diabetes and hypertensive with irregular treatment could add on to immunosuppressive state leading to infection and was treated with Meropenem and Cotrimoxazole following which patient clinically improved. Thus a holistic approach with clinicians and microbiologists plays a crucial role in the treatment of Melioidosis”
Introduction
Among all the zoonotic disease, Melioidosis which is caused by Burkholderia pseudomallei has gained importance because of its ubiquitous nature. In 1911 in Burma, Captain A. Whitmore a British pathologist along with C.S. Krishnaswami, an assistant surgeon, were curious about a human infective disease which mostly resembled glanders but was also easily distinguishable, especially in malnourished, neglected people. On autopsy of the patients, these lesions were mainly in the lungs, having peculiar caseous consolidations very different from lobar pneumonia / tuberculosis in their distribution and also in their appearance. This was also seen in liver, spleen and kidneys. They also found that in pure culture, exclusive Gram-negative motile rods much different from the glanders bacterium was observed. Whitmore named the etiological agent as Bacillus pseudomallei. In experimental animals the same lesions were reproduced, and the same was observed in 38 other patients. There was an outbreak of septicemic disease among laboratory animals especially in Malaya. Stanton and Fletcher named the new disease as Melioidosis.[1]
It is a Gram negative, motile, aerobic bacteria, oxidase positive, with polar flagella (occasional) and bipolar safety pin appearances which is better observed in staining. It grows easily on standard bacterial culture medium. In endemic region the organism is particularly present in soil and water surface. Humans acquire the infection by inhalation, contact with contaminated soil especially through skin abrasions or ingestion. Occasionally laboratory acquired infections do occur, but person to person and zoonotic infection are uncommon. [2] Global modeling estimated 1 lakh melioidosis cases around 2015, resulting in 54% mortality rate.
Mostly, the disease was recent in origin but latency can also be observed, hence called as “Vietnam time bomb” disease. B. pseudomallei is known to affect all cell types. This nature of tropism at cellular level contributes its adaptability as a pathogen and its broad range of symptoms. Patients who were predisposed to severe disease usually have underlying conditions such as diabetes and renal disease, describing as an opportunistic agent. There is no clarity in the seroprevalence of general population, particularly in endemic regions. Perhaps the most questioning aspect of the disease is the long incubation period in recrudescent melioidosis. In all of these cases, underlying medical conditions were present at the time of reactivation. This raises a series of questions regarding the pathogenesis of disease caused by the bacterium. [3]
Melioidosis gained attention globally since Burholderia pseudomallei was considered as an agent for terrorism and biological warfare by Centers for Disease Control and Prevention (CDC). It is prevalent in Australia, Thailand and Indian subcontinents with many other Southeast Asian countries which has been endemic for Melioidosis. In India, cases has been reported from Tamil Nadu, Karnataka, Kerala and Pondicherry. Associated factors with disease acquisition in include size and route of the inoculum, extreme weather conditions and host defence mechanism. [4] B. pseudomallei genome has two chromosomes, the larger carries genetic elements involving core physiological functions, while smaller chromosome carries genes associated with accessory functions, such as adaptation to different environments encoding several virulence factors like quorum sensing, the capsular polysaccharide and type III secretion systems,. The chromosomes highly dynamic and the extensive genetic diversification observed over time in a single individual. The portion of the genome which has high variability between strains contribute to virulence and antibiotic resistance. [5] B. pseudomallei is facultative intracellular organism that adhere, invade, survive and replicate within pulmonary phagocytic and epithelial cells. Critical step involves the establishment of infection predominantly by cell adhesion. For B. pseudomallei, cell adhesion is mediated by proteins (specific membrane) such as extracellular adherence protein, adhesion and flagellin that are regulated by temperature. [6]
Transmission is predominantly by contact with contaminated soil and water especially through penetrating wounds/ skin abrasions, ulcers/ burns. Transmission also occurs through inhalation. Farmers are more prone as they are working in paddy fields and are frequently exposed to B. pseudomallei-contaminated soil and water through skin inoculation. Infections are more prone in rainy season and people of all age groups are susceptible, but adults with predisposing conditions were mostly affected. The case fatality rate (CFR) may reach up to 40% if left untreated. Carbapenems (imipenem, meropenem), Ceftazidime, Amoxicillin–clavulanate, Piperacillin, Cephalosporins, showed various degrees of bactericidal activity. Treatment for intensive therapy to be given for at least 14 days. The minimum treatment durations for melioidosis with osteomyelitis, central nervous system infection, and deep-seated abscess were six, eight and four weeks, respectively. Subsequent eradication therapy has been suggested to prevent recrudescence or relapse of melioidosis. Oral trimethoprim-sulfamethoxazole was preferred antibiotic and the duration of treatment was three to six months.
Melioidosis is an emerging infection in India and it should be kept in mind as differential diagnosis when there are features suggestive of tuberculosis, hence it is proposed to highlight the clinical presentation, diagnosis, treatment and outcome of this dreaded disease. [7]
Case Presentation
A female patient, 45 years old, came with complaints of high grade temperature associated with chills since 1 ½ months. History of vomiting (multiple episodes) immediately after eating associated with anorexia. Patient had history of acute lower abdominal pain, more intensely in the upper left quadrant area. She was doing agriculture based occupation for the past 29 years, and was on mixed diet. A Known case of diabetes and hypertensive for the past 7 years. On examination general condition was fair, febrile, blood pressure 100/70, Pulse rate130/minute, SpO2 97% cardiovascular system, respiratory system normal, per abdomen soft, diffuse tenderness present, no free fluid in the abdomen and splenomegaly present
Investigations
Total count: 15,300/microliter
Hemoglobin: 9.2g/dl (peripheral smear: Microcytic hypochromic anemia).Neutrophilic predominance, Thrombocytopenia observed
Total protein: 7.8gldl
Albumin: 2.1 g/dl
Sodium: 116.0meq/L, Potassium: 4.6meq/L
Random blood sugar: 300 mg/dl, viral markers were negative, Dengue - negative, Widal test- negative, ANA- negative. RA-Negative, CK, CKMB enzymes were within normal limits.
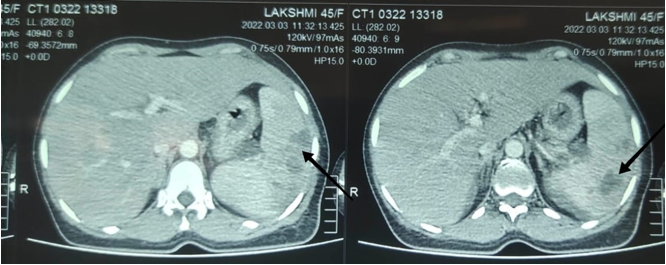
CT Abdomen: Multiple well defined peripheral enhancing lesion noted involving spleen-largest measuring 4*3 cm in inferior pole showing multiple splenic abscesses. ([Figure 4])
Ultra sonogram: Splenomegaly with multiple cystic areas noted.USG guided pigtail aspiration was done and sent for microbiological investigations.
Macroscopic slide agglutination test (MSAT) - 2+ (Leptospira)
Microbiological workup
Direct Gram stain of the purulent aspirate revealed numerous pus cells, Gram negative bacilli seen (safety pin appearance).
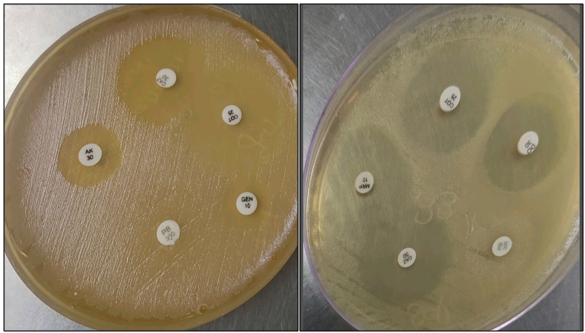
Cultured on standard bacteriological media. MacConkey agar showed rough, corrugated non lactose fermenting colonies, Blood agar showed rough grey colonies. Biochemical parameters was observed, it was a no fermenter and Oxidase test was positive and motility testing by LIA found the organism to be motile, which was identified as Burkholderia pseudomallei with the conventional techniques. Identification was confirmed by VITEK2 MS. Antimicrobial susceptibility was performed which showed Sensitive to Ceftazidime, Cotrimoxazole, Meropenem, Imipenem, Piperacillin, Tetracycline, Doxycycline, Amoxicillin-clavulanate. Resistant to Amikacin, Gentamicin, Polymyxin B (intrinsic resistant) ([Figure 3])
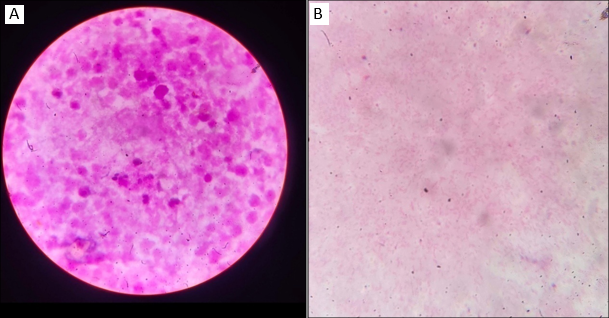
Blood culture showed no growth and that was reported Gram staining of the pus sample showing numerous pus cells and Gram negative bacilli seen ([Figure 1]A,B)
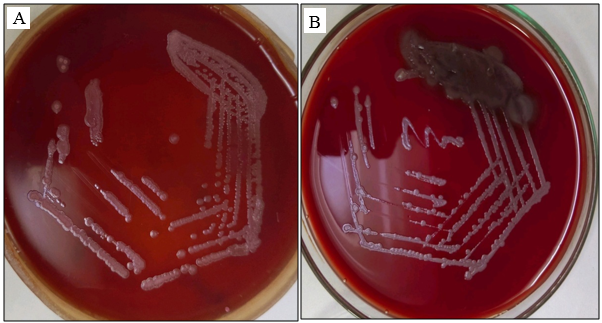
In [Figure 2] MacConkey agar showing rough non lactose fermenting colonies. Blood agar showing rough greyish colonies.
Discussion
Mode of transmission can be ingestion, inhalation and also contact with soil especially through skin abrasions. Proportion of males having the infection was more common in most of the case series analyzed preferably because of their work which is mostly outdoor in nature.
Our case was a female patient doing agriculture as principal occupation. Melioidosis has got widespread occurrence and predominantly found in soil of almost every states of India. Prevalence is more in southern states. India's rural population who lives in proximity to agricultural land is quite susceptible to this neglected killer disease. But it is underreported because of: (i) Lack of education about the disease (ii) decreased suspicion rate and (iii) under-recognized. Microbiologist should suspect on isolation of a gram negative bacillus, bipolar stained and oxidase positive, which polymyxin resistant. [8]
The patient is a chronic case of diabetes and hypertensive with irregular treatment stating that could add on to the immunosuppressive state and leading to infection. [9], [10], [11], [12] Diabetes mellitus is the strongest well-established risk factor. Other risk factors include chronic kidney or lung disease, liver disease, and heavy alcohol use, estimated 20% of infections occur in patients who do not have specified risk factors.
Melioidosis cases are considered seasonal and raise during the rainy season. Both leptospirosis and melioidosis are zoonotic found in soil and water. Co-infection is expected in people who have exposure to fresh water and soil.
Risk factors of patients with splenic abscess includes Diabetes mellitus (20.5%), chronic kidney disease (7.7%), Human immunodeficiency virus (2.6%) and clinical features include history of pyrexia (97.4%), abdominal pain (5%), high white cell count. [13]
Total serum electrolytes (sodium-low) in this patient. Hyponatremia is the most common finding in hospitalized patients with worst clinical outcomes.
B. pseudomallei affects almost all parts of the body causing asymptomatic infection, abscesses in visceral organs landing in septic shock. Here, patient presented with multiple spleen abscess. Splenic abscess is the most common, followed by liver and kidney abscesses. [14], [15], [16]
Disseminated cases most commonly causes visceral abscesses predominantly involving liver, spleen, or prostate. In areas where the prevalence is high, multiple hepatic and splenic abscesses along with CT necklace sign were predominantly suggestive of melioidosis. Intraabdominal abscesses were larger compared to non-melioidosis intraabdominal abscesses. A splenic abscess can rupture in rare cases, and splenic vein thrombosis can also occur. The presence of a splenic abscess that too in a diabetic patient who has severe sepsis should have a prompt suspicion of melioidosis, and empirical antimicrobial therapy should be considered.
Almost half of the patients with melioidosis, presents with pneumonia and this is the most common clinical presentation. Pneumonia can be the primary clinical manifestation or it can occur secondarily to the initial phase at a distant foci. Acute pulmonary stage is characterized by diffuse nodular infiltrates that coalesce and form a cavity, with the upper lobes frequently involved. CT usually reveals bilateral disseminated miliary nodules with necrotizing pneumonia. Melioidosis lung nodules are typically larger and irregular. In pulmonary tuberculosis, lung nodules are often smaller. Bacteremic melioidosis carries worst prognosis in comparison to non-bacteremic melioidosis. The presence of septic shock is a strong predictor of death. The mortality rate for bacteremic melioidosis was estimated to be 37%, compared to 4% in patients with melioidosis without bacteremia. Positive blood culture in the second week of hospitalization is a strong prognostic factor for mortality. These evidences supports the need for follow up blood cultures in admitted patients. Treatment failure may occur during Ceftazidime regimen due to potential resistance via penicillin-binding protein 3 gene deletions, lack of growth of resistant strains on culture plates, and early bacteremia relapse. Meropenem is thus preferred over ceftazidime in the treatment of persistent bacteremia and also in critically ill patients. [17]
Culture of appropriate clinical specimens is the standard criterion for the diagnosis of melioidosis and the organism can usually readily be cultured. In our case organism was identified from pus aspirated from spleen. [18] Blood culture was negative. At present, the drug of choice for melioidosis is intravenous ceftazidime 2 g intravenously every 8 hours and in combination with oral cotrimoxazole (8/40 mg/kg/day up to 320/1600 mg/day) in divided doses every 12th hour for 10 to 14 days. In simple bacteremic melioidosis, ceftazidime alone can be given in the intensive phase for 10 to 14 days. In our case also antimicrobial sensitivity pattern showed high degree of susceptibility to Ceftazidime, Cotrimoxazole, Meropenem, and patient was treated with meropenem and cotrimoxazole and clinically improved, discharged in good condition. Although vaccine development under research, currently no vaccines are available to prevent B. pseudomallei infection. [19] Melioidosis is a burdensome disease to diagnose clinically because of diverse presentations and lack of knowledge. Melioidosis is considered a latent disease. Always having suspicion, early use of proper antibiotics and systematic clinical support will improve net result. Patients with shock have a very low survival rate in spite of appropriate treatment. [20]
Conclusion
Melioidosis challenges to both laboratory scientist and physician. Initially diagnostic techniques weren’t available much to differentiate. No bacteriological interpretation was possible in the very first case. The Gram negative bacillus isolated from blood culture didn't survive long enough for definitive identification and the one surviving case had no positive culture but a delayed seroconversion. Isolation of organisms in pure culture was done only in later cases. There should be broad knowledge of the various clinical presentations of melioidosis and accurate diagnostic methods will result in quicker diagnosis and a better outcome. At the end, effective appropriate antibiotic therapy is to be made available for this potentially deadly infection. This will reduce the mortality and help prevent relapse of this disease. Melioidosis is a disease which has public health importance. Sporadic cases suggest that there may be foci of infection that is missed. It should always be kept as a differential diagnosis in patients who have agriculture based occupation due to its saprophytic nature. Due to the wide spectrum in presentation and long latency period, the diagnosis is challenging to many clinicians. So multidisciplinary approach involving clinicians, microbiologists should always be considered in arriving at diagnosis and management of Melioidosis.[20], [21]
Source of Funding
None.
Conflict of Interest
None.
References
- Yabuuchi E, Arakawa M. Burkholderia pseudomallei and melioidosis: be aware in temperate area. Microbiol Immunol. 1993;37(11):823-36. [Google Scholar]
- Patro S, Panda S, Mishra D. Burkholderia Infections in Diabetic Section Patients Emerging as a Challenge for Physicians: A Case Series. J Clin Diagn Res. 2019;13(3):4-7. [Google Scholar]
- Gan Y. Interaction between Burkholderia pseudomallei and the Host Immune Response: Sleeping with the Enemy?. J Infect Dis. 2005;192(10):1845-50. [Google Scholar]
- Wiersinga W, Poll TVD, White N, Day N, Peacock S. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4(4):272-82. [Google Scholar]
- Hemarajata P, Baghdadi J, Hoffman R, Humphries R. Burkholderia pseudomallei: Challenges for the Clinical Microbiology Laboratory. J Clin Microbiol. 2016;54(12):2866-73. [Google Scholar]
- Mariappan V, Vellasamy K, Barathan M, Girija A, Shankar E, Vadivelu J. Hijacking of the Host's Immune Surveillance Radars by Burkholderia pseudomallei. Front Immunol. 2021;12. [Google Scholar] [Crossref]
- Lin C, Chen T, Lu P, Lin W, Chen Y. Melioidosis presenting with isolated splenic abscesses: a case report. Kaohsiung J Med Sci. 2007;23(8):417-21. [Google Scholar]
- Pandey V, Rao S, Rao S, Acharya K, Chhabra S. Burkholderia pseudomallei musculoskeletal infections (melioidosis) in India. Indian J Orthop. 2010;44(2):216-20. [Google Scholar]
- RM, Baby P, Kumar A, Surendran S, Pradeep M, Rajendran A. Risk Factors for Mortality in Melioidosis: A Single-Centre, 10-Year Retrospective Cohort Study. ScientificWorldJournal. 2021. [Google Scholar] [Crossref]
- Kunnathuparambil S, Sathar S, Tank D, Sreesh S, Mukunda M, Narayanan P. Splenic abscess due to chronic melioidosis in a patient previously misdiagnosed as tuberculosis. Ann Gastroenterol. 2013;26(1):77-9. [Google Scholar]
- Chowdhury S, Barai L, Afroze S, Ghosh P, Afroz F, Rahman H. The Epidemiology of Melioidosis and Its Association with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Pathogens. 2022;11(2). [Google Scholar] [Crossref]
- Kronsteiner B, Chaichana P, Sumonwiriya M, Jenjaroen K, Chowdhury F. Diabetes alters immune response patterns to acute melioidosis in humans. Eur J Immunol. 2019;49(7):1092-6. [Google Scholar]
- Chang C. Burkholderia Pseudomallei As The Predominant Cause Of Splenic Abscess In Kapit, Sarawak, Malaysian Borneo. J Ayub Med Coll Abbottabad. 2023;35(2):348-50. [Google Scholar]
- Wuthiekanun V, Limmathurotsakul D, Chantratita N, Feil E, Day N, Peacock S. Burkholderia Pseudomallei is genetically diverse in agricultural land in Northeast Thailand. PLoS Negl Trop Dis. 2009;3(8). [Google Scholar] [Crossref]
- Cheng A, Currie B. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18(2):383-416. [Google Scholar]
- Currie B, Ward L, Cheng A. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. Nov. 2010;4(11). [Google Scholar] [Crossref]
- Yik C, Lee H. A Fatal Case Of Persistent Burkholderia Pseudomallei Bacteraemia With Severe Pneumonia And Splenic Abscess. J Ayub Med Coll Abbottabad. 2023;35(2):331-3. [Google Scholar]
- Dance D. Melioidosis as an emerging global problem. Acta Trop. 2000;74(2-3):115-9. [Google Scholar]
- Phillips E, Garcia E. Burkholderia pseudomallei. Trends Microbiol. 2023;32(1):105-6. [Google Scholar]
- Ramamoorthi K, Saravu K, Mukhopadhyay C, Shastry B. Melioidosis: An underdiagnosed disease in India (epidemiology, clinical features, and outcomes). Asian Biomed. 2013;7(2):249-56. [Google Scholar]
- Inglis T, Rolim D, Rodriguez J. Clinical guideline for diagnosis and management of melioidosis. Rev Inst Med Trop Sao Paulo. 2006;48(1):1-4. [Google Scholar]
Article Metrics
- Visibility 9 Views
- Downloads 2 Views
- DOI 10.18231/pjms.v.15.i.1.235-239
-
CrossMark
- Citation
- Received Date July 13, 2022
- Accepted Date December 01, 2023
- Publication Date March 13, 2025